User login
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
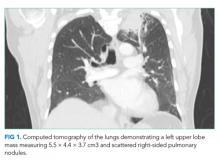
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
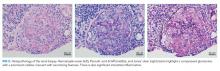
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
A 65-year-old man was transferred to a tertiary academic medical center with one week of progressive shortness of breath, dry cough, and fevers. He reported no weight loss or night sweats but had experienced mild right upper quadrant pain and anorexia for the preceding three weeks. Several years had passed since he had consulted a physician, and he did not take any medications. He immigrated to the United States from Mexico four decades prior. He traveled back frequently to visit his family, most recently one month before his presentation. He worked as a farming supervisor in the Central Valley of California. He smoked tobacco and had a 30 pack-year history. He drank alcohol occasionally and denied any drug use.
Causes of subacute cough and dyspnea include bronchitis, pneumonia, heart failure, and asthma. Pneumonia and heart failure might cause right upper quadrant pain from diaphragmatic irritation and hepatic congestion, respectively. Metastatic cancer or infection may lead to synchronous pulmonary and hepatic involvement. The patient is at increased risk of lung cancer, given his extensive smoking history.
The patient’s place of residence in the Southwestern United States places him at risk of respiratory illness from coccidioidomycosis. His exact involvement with animals and their products should be further explored. For example, consumption of unpasteurized milk might result in pneumonia, hepatitis, or both from M. bovis, Brucella species, or C. burnetii. His travel to Mexico prompts consideration of tuberculosis, histoplasmosis, and paracoccidiomycosis as causes of respiratory and possible hepatic illness.
Two weeks prior, the patient had initially presented to another hospital with one week of intermittent right upper quadrant pain unrelated to eating. An abdominal ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan were normal. Computed tomography (CT) of the chest, abdomen, and pelvis with contrast demonstrated a left upper lobe lung mass measuring 5.5 × 4.4 × 3.7 cm3 and scattered right-sided pulmonary nodules (Figure 1). He underwent CT-guided biopsy of the mass and was discharged with a presumed diagnosis of primary pulmonary malignancy with plans for outpatient follow-up.
Over the next four days, the patient developed progressive dyspnea with cough and subjective fevers. The patient was readmitted with a diagnosis of postobstructive pneumonia and acute kidney injury (creatinine increased from 0.7 mg/dL to 2.9 mg/dL between admissions), and this finding was attributed to contrast-induced nephropathy from his recent CT scan. He was treated with vancomycin and piperacillin/tazobactam for two days but wished to transfer to a tertiary care hospital for a second opinion.
Postobstructive pneumonia, pulmonary embolism, and pleural effusion are common causes of dyspnea in patients with lung cancer. The patient’s travel and occupational history, lung nodules, acute renal insufficiency, and rapidly progressive respiratory symptoms prompt consideration for radiographic mimickers of lung cancer. Tuberculosis might present as a lung mass (pulmonary tuberculoma) during primary infection or reactivation. Noninfectious causes of pulmonary masses and nodules include metastatic cancer (eg, colon cancer), sarcoidosis, IgG4-related disease, and granulomatous polyangiitis (GPA).
Contrast-induced nephropathy is unusual in patients with normal renal function. More probable explanations include hypovolemia or acute tubular necrosis (ATN) from underlying inflammation. The patient’s CT-negative right upper quadrant pain may be a distinct process or represent another facet of a disseminated illness such as hepatic infiltration from lymphoma.
Upon arrival, the patient’s temperature was 38°C, heart rate (HR) 107 beats per minute, blood pressure (BP) 159/89 mm Hg, respiratory rate 25 breaths per minute, and oxygen saturation 92% on 2 L of oxygen per minute. He showed no signs of distress. Mild scleral icterus was noted. The cardiac exam was normal. Auscultation revealed scattered wheezes and crackles in the left upper lobe. Mild right upper quadrant tenderness without hepatosplenomegaly was noted on the abdominal exam. The patient’s lower extremities exhibited bilateral trace edema. No rash was observed, and his neurologic exam was normal.
The white blood cell (WBC) count was 28,300 per cubic millimeter (87% neutrophils, 3.6% lymphocytes, and 0.03% eosinophils), hemoglobin 11.1 g per deciliter, and platelet count 789,000 per cubic millimeter. Sodium was 127 mmol per liter, potassium 4.6 mmol per liter, chloride 101 mmol per liter, bicarbonate 13 mmol per liter, blood urea nitrogen 60 mg per deciliter, and creatinine 3.4 mg per deciliter. Aspartate aminotransferase and alanine aminotransferase levels were normal. Alkaline phosphatase was 283 units per liter (normal range, 31-95), and total bilirubin was 4.5 mg per deciliter (normal range, 0.2-1.3) with a direct bilirubin of 2.7 mg per deciliter. Urinalysis demonstrated urine protein of 30 mg/dL, specific gravity of 1.013, negative nitrites, 10-21 white cells per high-powered field (normal, < 5), and 21-50 red cells per high-powered field (normal, < 3). Urine microscopy revealed muddy brown casts but no cellular casts or dysmorphic red cells. A chest radiograph (CXR) showed patchy consolidations in the bilateral upper lobes and left lower lobe along with Kerley B lines, a small left pleural effusion, and thickened right horizontal fissure; the left upper lobe mass was re-demonstrated. Vancomycin, piperacillin-tazobactam, and azithromycin were administered.
At this point, the most likely source of sepsis is multifocal pneumonia. The patient is at risk for S. aureus and P. aeruginosa given his recent hospitalization. A severe form of leptospirosis (Weil’s disease) is associated with pulmonary disease, hyperbilirubinemia, and renal failure. Repeat abdominal imaging is necessary to evaluate for cholangitis given the patient’s right upper quadrant pain, fever, and jaundice. It would also help categorize his cholestatic pattern of liver injury as intrahepatic or extrahepatic (eg, stricture). An infiltrative disease such as sarcoidosis may cause both intrahepatic cholestasis and parenchymal lung disease, although the pleural pathology is uncommon.
His normal cardiac exam does not exclude cardiogenic pulmonary edema, a common cause of interstitial edema and pleural effusion. In this setting of systemic inflammation (neutrophilia, thrombocytosis, and hypoalbuminemia), the thickened right horizontal fissure and interlobular septa might represent an infiltrative process, such as lymphangitic carcinomatosis, lymphoma, or sarcoidosis.
Muddy brown casts are characteristic of ATN. The patient’s risk factors for ATN include sepsis and previously administered iodinated contrast. Fluid retention from oliguric renal failure is likely contributing to his hyponatremia and lower extremity edema. Pathology isolated to the tubules, however, would not cause hematuria and pyuria and suggests glomerular or interstitial disease. The lack of cellular casts on a single urinary specimen does not eliminate the likelihood of either disease. Hematuria and diffuse parenchymal lung disease prompt consideration of pulmonary-renal syndromes, such as anti-glomerular basement membrane disease, GPA, and systemic lupus erythematosus, which can all be triggered by infection.
On the night of transfer, the patient experienced acute respiratory distress. Heart rate was 130 beats per minute, BP 170/95 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation 88% on six liters of supplemental oxygen by nasal cannula. His arterial blood gas demonstrated a pH of 7.23, PaCO2 of 32 mm Hg, and PaO2 of 65 mm Hg. He was emergently intubated for progressive hypoxemic respiratory failure. A small amount of blood was noted in the endotracheal tube. A noncontrast CT of the chest demonstrated multifocal airspace opacities and bilateral pleural effusions. The previously noted left upper lobe mass was unchanged.
Rapid respiratory decline and diffuse alveolar disease commonly result from aspiration, flash pulmonary edema, and acute respiratory distress syndrome (ARDS). Necrotizing pneumonia (eg, S. aureus) and trauma during intubation are possible causes of blood in his endotracheal tube. However, in the setting of multifocal airspace opacity, renal insufficiency, hematuria, and rapid respiratory decline, the blood might represent diffuse alveolar hemorrhage (DAH). Bronchoscopy with bronchioalveolar lavage to evaluate for pulmonary edema, infection, and hemorrhage would be indicated.
The patient subsequently developed oliguria, requiring continuous renal replacement therapy. An echocardiogram demonstrated impaired left ventricular relaxation and a reduced ejection fraction of 45% without segmental wall motion abnormalities or valvular disease, and a right ventricular systolic pressure of 36 mm Hg. Over the next 12 hours, his respiratory status improved, and he was extubated to 15 L per minute of supplemental oxygen by high-flow nasal cannula (HFNC).
The pathology report of the lung biopsy from the other hospital disclosed chronic inflammation and fibrosis with ill-defined areas of necrosis and myxoid degeneration surrounded by nuclear palisading suggestive of granulomatous inflammation. Staining for acid-fast bacilli (AFB) and fungal organisms was negative.
The rapid pulmonary recovery is inconsistent with multifocal pneumonia or ARDS. Flash pulmonary edema might result in sudden hypoxemic respiratory failure that resolves with positive pressure ventilation and ultrafiltration. However, this condition would not explain the biopsy results. Granulomatous lung pathology often results from mycobacterial or fungal disease. Tuberculosis and fungal pneumonia are not excluded with negative staining alone. However, neither would cause self-limited respiratory failure. Histologic evidence of necrosis lessens the likelihood of sarcoidosis, which rarely causes fulminant pulmonary disease. Lymphoma can result in granulomatous inflammation but would not cause transient pulmonary disease. GPA, a cause of necrotizing granulomatous lung disease, might result in a lung mass and worsened hypoxemia through DAH.
The patient continued to require 15 L of oxygen per minute by HFNC. He had persistent bilateral perihilar alveolar and interstitial opacities on CXR. Repeat WBC count was 29,200 per cubic millimeter, hemoglobin 7.8 g per deciliter, and platelets 656,000 per cubic millimeter. The C-reactive protein was 300 mg per L (normal range, <6.3) and erythrocyte sedimentation rate 100 mm per hour (normal range, <10). Legionella urinary antigen, serum immunodiffusion for Coccidiodes imitus, human immunodeficiency virus antibody, respiratory viral panel, and beta-D glucan were negative. Rare acid-fast bacilli were visualized in one out of three concentrated AFB sputum smears. He was started on empiric antituberculous therapy with rifampin, isoniazid, pyrazinamide, and ethambutol.
The sputum sample is suggestive of pulmonary tuberculosis. The salient features of this case include systemic inflammation, pulmonary nodules and mass, necrotizing granulomatous lung pathology, renal insufficiency, and hematuria. Disseminated tuberculosis might explain all these findings. However, a positive AFB smear may signal the presence of a nontuberculous mycobacteria, which is less likely to cause this clinical syndrome.
M. tuberculosis complex polymerase chain reaction (MTB PCR) assay returned negative for M. tuberculosis. Antiproteinase 3 antibody was 1,930 units (normal range, <20). Antimyeloperoxidase and antiglomerular basement membrane antibodies were negative.
Tuberculosis and GPA share several overlapping features, such as necrotizing lung pathology and less commonly antineutrophil cytoplasmic autoantibody (ANCA)-associated antibodies. However, the lung mass, acute renal and respiratory failure, hematuria, and the degree of anti-proteinase 3 level elevation are highly suggestive of GPA. The negative MTB PCR raises the possibility that a nontuberculous mycobacterium was detected on the sputum smear. Nevertheless, continued treatment until finalization of culture results is appropriate given that tuberculosis is endemic in Mexico.
The patient’s presenting features of right upper quadrant tenderness, jaundice, and cholestatic hepatitis remain poorly explained by either of these diagnoses. Neither tuberculosis nor GPA commonly presents with accompanying hepatic involvement, though both have been occasionally described as causing hepatitis. As the greatest concern in this patient remains his progressive renal failure and accompanying pulmonary hemorrhage, a renal biopsy to assess for glomerulonephritis associated with GPA is warranted before further investigation into the cause of his cholestatic hepatitis.
A core renal biopsy demonstrated pauci-immune focal crescentic and necrotizing glomerulonephritis with mixed tubulointerstitial inflammation (Figure 2). In conjunction with the pulmonary syndrome and positive antiproteinase 3 serology, a diagnosis of granulomatosis with polyangiitis was made. The patient was treated with pulse dose steroids, rituximab, and plasma exchange. Two weeks later, the sputum mycobacterial culture returned positive for Mycobacterium llatzerense and anti-tuberculous treatment was discontinued.
Over the following weeks, the patient improved and was transitioned off dialysis prior to hospital discharge. By six months later, he had resolution of his hemoptysis, shortness of breath, liver biochemical test abnormalities, and significant improvement in his renal function. Repeat sputum mycobacterial cultures were negative.
DISCUSSION
A 65-year-old man from Mexico with a significant smoking history presented with an apical lung mass and cough, prioritizing tuberculosis and pulmonary malignancy. As the case unfolded, renal failure, multifocal lung opacities, conflicting tuberculosis test results, positive anti-proteinase 3 antibody, and ultimately a renal biopsy led to the diagnosis of granulomatosis with polyangiitis (GPA).
The correct interpretation of occasionally conflicting mycobacterial testing is crucial. Mycobacterial cultures remain the gold standard for diagnosing tuberculosis. However, results take weeks to return. Rapid tests include acid-fast bacilli (AFB) smear microscopy and nucleic acid-amplification tests (NAAT) of sputum or bronchoalveolar samples.1 When three sputum smears are performed, the sensitivity of AFB smear microscopy for tuberculosis in immunocompetent hosts is 70%.1 The AFB smear does not distinguish between different mycobacterial organisms. Thus, a positive result must be interpreted with the relative prevalence of tuberculosis and nontuberculous mycobacteria (NTM) in mind. The addition of NAAT-based assays has allowed for enhanced sensitivity and specificity in the diagnosis of tuberculosis, such that a negative NAAT in a patient with a positive AFB smear strongly argues for the presence of a NTM.2-4
NTM are widely prevalent environmental microbes, with over 140 species described, and careful consideration is required to determine if an isolate is pathogenic.5 Given their ubiquitous nature, a high rate of asymptomatic respiratory and cutaneous colonization occurs. Correspondingly, the diagnosis of NTM disease requires multiple positive cultures or pathologic features on tissue biopsy, compatible clinical findings, and diligent exclusion of other causes.5 A retrospective study of all NTM isolates in Oregon from 2005-2006 revealed that only 47% of patients met the guideline criteria for having symptomatic NTM disease.6 In our case, the patient’s sputum grew M. llatzerense, an aerobic, nonfermenting mycobacterium found in water sources that has only infrequently been implicated as a human pathogen.7,8 Subsequent AFB sputum cultures were negative, and serial imaging showed resolution of the pulmonary findings without additional antimycobacterial therapy, suggesting that this organism was not responsible for the disease process.
Along with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), GPA is an antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis that predominantly affects small to medium sized vessels. Although it can occur at any age, GPA most commonly afflicts older adults, with men and women being diagnosed at roughly equal rates.9 GPA is a multisystem disease with a wide array of clinical manifestations. The most frequently involved sites of disease are the respiratory tract and kidneys, although virtually any organ can be affected. Sino-nasal disease, such as destructive sinusitis, or ear involvement are nearly universal. Lower respiratory manifestations occur in 60% of patients, but are highly diverse and reflect the inherent difficulty in diagnosing this condition.9-11 Additionally, GPA is a frequent cause of the pulmonary-renal syndromes, with glomerulonephritis occurring in 80% of patients.9
The diagnosis of GPA in this case was delayed, in part, due to features suggestive of malignancy and pulmonary tuberculosis. While sino-nasal disease was not noted during this hospitalization, the patient had many different respiratory manifestations, including a dominant pulmonary mass, diffuse nodules, and hypoxemic respiratory failure due to suspected diffuse alveolar hemorrhage (DAH), all of which have been reported in GPA.12 Dysmorphic red cells and red blood cell casts are not sensitive for renal involvement in GPA; their absence does not exclude the possibility of an ANCA-associated vasculitis.13 Hematuria and rapid progression to oliguric renal failure are characteristic of a vasculitic process and should sway clinicians away from a working diagnosis of ATN.
The diagnosis of GPA involves the synthesis of clinical data, radiographic findings, serologic testing, and histopathology. ANCA testing is an essential step in the diagnosis of GPA but has limitations. Patients with GPA more commonly have ANCAs targeting the enzyme proteinase-3 (PR3-ANCA), with MPA being more closely associated with myeloperoxidase (MPO-ANCA), although cross-reactivity and antibody-negative disease can occur.14 Although 90% of patients with GPA with multiorgan involvement will have a positive ANCA, a negative test is more common in localized upper airway disease, where only 50% have a positive ANCA.15 A number of drugs, medications, infections, and nonvasculitic autoimmune diseases have been associated with positive ANCA serologies in the absence of systemic vasculitis.14,16,17 As such, pathologic demonstration of vasculitis is necessary for establishing the diagnosis. Typical sites for biopsy include the kidneys and lungs.9
This case illustrates how clinicians often find themselves at a diagnostic crossroads—being forced to choose which clinical elements to prioritize. At various points, our patient’s presentation could have been framed as “a man from a Tb-endemic country with hemoptysis and an apical opacity,” “an elderly man with extensive smoking history and lung mass,” or “a patient with elevated inflammatory markers and pulmonary-renal syndrome”. In such situations, it is incumbent on the clinician to evaluate how well a given problem representation encompasses or highlights the salient features of a case. As with painting or photography, an essential aspect of appreciating the whole picture involves carefully selecting the right frame.
KEY TEACHING POINTS
- The diagnosis of tuberculosis relies on smear microscopy, nucleic-acid amplification testing (NAAT), and cultures. A positive AFB smear with negative NAAT suggests the presence of a nontuberculous mycobacteria (NTM).
- NTM are common environmental organisms and often exist as nonpathogenic colonizers.6 The diagnosis of NTM disease requires exclusion of other causes and careful clinical, microbiologic, and radiographic correlation.
- Granulomatosis with polyangiitis is a multisystem disease often involving the respiratory track and kidney. Pulmonary disease can present with airway involvement, parenchymal nodules, opacities, pleural findings, and diffuse alveolar hemorrhage.12
Disclosures
Drs. Minter, Geha, Boslett, Chung, and Ramani have no disclosures. Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME).
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
1. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. PubMed
2. Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):Cd009593. PubMed
3. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus afb smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081-1088. PubMed
4. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015. PubMed
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed
6. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. PubMed
7. Teixeira L, Avery RK, Iseman M, et al. Mycobacterium llatzerense lung infection in a liver transplant recipient: case report and review of the literature. Am J Transplant. 2013;13(8):2198-2200. PubMed
8. Cárdenas AM, Gomila M, Lalucat J, Edelstein PH. Abdominal abscess caused by Mycobacterium llatzerense. J Clin Microbiol. 2014;52(4):1287-1289. PubMed
9. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512-1523. PubMed
10. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72(6):1003-1010. PubMed
11. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257-266. PubMed
12. Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912. PubMed
13. Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant. 2018;33(8):1397-1403. PubMed
14. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463-473. PubMed
15. Borner U, Landis BN, Banz Y, et al. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener’s granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26(6):475-480. PubMed
16. Mahr A, Batteux F, Tubiana S, et al. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66(6):1672-1677. PubMed
17. Sherkat R, Mostafavizadeh K, Zeydabadi L, Shoaei P, Rostami S. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran J Immunol. 2011;8(1):52-57. PubMed
© 2019 Society of Hospital Medicine