User login
Social Disadvantage, Access to Care, and Disparities in Physical Functioning Among Children Hospitalized with Respiratory Illness
Examining disparities in health-related quality of life (HRQoL) outcomes in children provides a unique patient-centered perspective on pediatric health services equity.1,2 Prior studies have demonstrated the relationship between minority race, low socioeconomic status, and lower maternal education with poorer HRQoL outcomes in children.3-6 Some studies have also shown a dose-response relationship between social disadvantage markers and poorer child health status.7,8 Furthermore, the associations between social disadvantage and poor access to care,9-11 and between poor access to care and lower HRQoL, are also well established.12-14
Examining HRQoL before and after hospitalization can further our understanding of how disparities in HRQoL may change once children engage with the medical system for an acute illness.15 Children requiring hospitalization constitute a useful population for examination of this question as they represent a group of children with variable social disadvantage markers and access to outpatient care.16 Although interventions to address social determinants of health for patients with social disadvantages have been associated with within-group improvements in HRQoL, none have assessed changes in disparities as an outcome.17 Furthermore, many of these studies were conducted in the outpatient setting,18,19 whereas hospitalization provides an additional point of care to address the social determinants of health for vulnerable families.20 Even for short hospitalizations, the 24/7 nature of hospital care provides the opportunity for frequent interactions with clinicians, nurses, and support staff to clarify illness-related questions, discuss other health concerns and unmet needs, and connect with social services or community resources. These opportunities may be particularly important for families with a higher number of social disadvantage markers and even more beneficial to those with difficulty accessing needed care from their primary medical home.
In this study, we focused on children with common respiratory illnesses (asthma, bronchiolitis, and pneumonia), which constitute the majority of childhood hospitalizations.21 Additionally, we only focused on the child’s physical functioning component of HRQoL because this component is most likely to improve after hospitalization for children with an acute respiratory illness.22 A prior study examining HRQoL before and after hospitalization demonstrated that most children return to and/or surpass their baseline physical functioning by 1 month after hospital discharge.23
Our primary objective was to examine associations between several markers of social disadvantage, access to care, and child physical functioning before and after hospitalization for acute respiratory illness. Second, we aimed to understand if access to care (defined as perceived difficulty/delays getting care) acts as an independent predictor of improvement in physical functioning from baseline to follow-up and/or if it modifies the relationship between social disadvantage and improvement in physical functioning (Appendix Figure).
METHODS
Study Design and Population
This study was nested within a multicenter, prospective cohort study of children who were hospitalized for asthma, bronchiolitis, or pneumonia between July 2014 and June 2016 at one of five children’s hospitals in the Pediatric Research in Inpatient Settings Network.24
We approached families for study participation within 72 hours of admission to the hospital using a standard protocol. Patients and their caregivers were eligible to participate in the study if the patient was 2 weeks to 16 years old and if the primary caregiver’s preferred language for medical communication was either English or Spanish. Patients with chronic medical conditions (except asthma), with moderate to severe developmental delay, with a history of prematurity <32 weeks, or who received care in the intensive care unit were excluded. Patients could only participate in the study once.
The study team set out to enroll an even number of patients across all three conditions. If a patient’s discharge diagnosis differed from their admission diagnosis (eg, from bronchiolitis to pneumonia), discharge diagnosis was used for condition group assignment. If the discharge diagnosis was not one of these three respiratory conditions, we excluded the patient from further analysis.
Data Collection
We collected data using two surveys. The first survey was administered within 72 hours of admission. This survey asked questions related to (1) caregiver-reported markers of social disadvantage, (2) caregiver perceptions of access to care, and (3) caregiver- and patient-reported assessments of physical functioning. The second survey was administered within 2 to 8 weeks after the patient’s discharge and included a second assessment of physical functioning.
Social Disadvantage
Patients were considered to have a marker of social disadvantage if their caregiver reported (1) being of non-White race and/or Hispanic ethnicity, (2) primarily speaking a language other than English at home and not speaking English very well (ie, limited English proficiency), (3) attaining at most a high school or equivalent degree, or (4) having a =/<$30,000 annual household income.
Access to Care
We used the following survey item from the 2009-2010 National Survey of Children with Special Health Care Needs25 to measure caregiver perceptions of access to care: “In the last six months, did you have any difficulties or delays getting care for your child because there were waiting lists, backlogs, or other problems getting an appointment?” We narrowed the original assessment time frame from 12 months to 6 months to provide a more proximal assessment of access in relation to the hospitalization.
Child Physical Functioning
We assessed child physical functioning using the physical functioning domain of the Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales and PedsQL Infant Scales, which have been validated for use in the inpatient setting.22 Caregivers completed one of these scales based on their child’s age. Assenting patients 8 to 16 years old completed the self-report PedsQL 4.0 Generic Core Scales instrument. When completing the first PedsQL survey, caregivers and patients reflected on the previous month before their child (or they) became ill to obtain a baseline physical functioning assessment.23 When completing the second PedsQL survey, caregivers and patients reflected on the past 7 days to obtain a follow-up assessment.
All study procedures were approved by the Western Institutional Review Board (IRB) or the participating hospitals’ IRB.
Statistical Analysis
Patients with no missing data for all four social disadvantage markers were categorized based on the number of markers they reported: none, one, two, or three or more markers. We combined patients with three and four social disadvantage markers into one group to maximize power for the analyses. We dichotomized the access to care variable and coded response options as “no difficulty/delays accessing care” if the caregiver chose “Never” and “any difficulty/delays accessing care” if they chose “Sometimes/Usually/Always.”
For each patient–caregiver dyad, PedsQL items were scored using a standard method in which higher scores reflected better functioning.22 A single set of PedsQL scores was used for each patient–caregiver dyad. We used self-reported patient scores if the patient completed the PedsQL instrument; otherwise, we used proxy-reported caregiver scores. Intraclass correlations between child self-report and parent proxy-report demonstrate moderate to good agreement above age 8 years.26 We computed a change in the physical functioning score by subtracting the baseline score from the follow-up score. The minimal clinically important difference (MCID) for the PedsQL instrument is 4.5 points, which we used to identify clinically meaningful differences.13
Analysis of variance models were constructed to test for differences in mean baseline and follow-up PedsQL scores (dependent variable) between the following independent variables: (1) social disadvantage groups and (2) those who reported having any difficulty/delays accessing care compared with those who did not. Only patient–caregiver dyads with both baseline and follow-up assessments were included in these analyses. Mixed-effects linear regression models were constructed to identify clinically meaningful differences in PedsQL scores between groups (MCID =/> 4.5) with adjustment for patient age, gender, respiratory condition, days between surveys, and hospital site as fixed effects. Site-specific random effects were included to account for within-hospital clustering. A similarly adjusted mixed-effects linear regression model was constructed to examine whether having any difficulty/delays accessing care modified the association between social disadvantage and PedsQL change scores (eg, an improvement from baseline to follow-up).
Because 17% of respondents had missing data for at least one social disadvantage marker, sensitivity analyses were conducted using multiple imputation to account for missing social disadvantage markers using chained equations.27 Sensitivity analyses were also conducted to adjust for severity of illness using vital sign data within the first 24 hours, which could only be validly captured on patients with asthma within our dataset. By restricting this latter analysis to patients with asthma, we were able to examine the relationships of interest in a population with chronic disease.
RESULTS
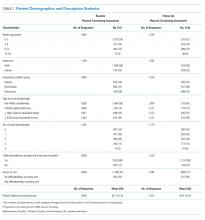
The study sample included 1,860 patients, of which 1,325 had both baseline and follow-up PedsQL data (71%). Descriptive statistics were similar between those who completed the baseline and follow-up surveys (Table 1).
Twenty-two percent of patients had >/=3 social disadvantages and 30% of caregivers reported having any difficulty/delays accessing care. The mean follow-up PedsQL score was higher than the baseline score (90.4 vs 82.5; Table 1).
Social Disadvantage Markers and PedsQL Scores
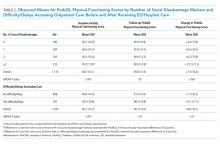
The number of social disadvantage markers was inversely related to mean baseline PedsQL scores, but there was no difference in mean follow-up PedsQL scores between social disadvantage groups (Table 2). In adjusted analyses, the mean baseline PedsQL score was −6.1 points (95% CI: −8.7, −3.5) lower for patients with >/= 3 social disadvantage markers compared with patients with no social disadvantage markers, which exceeded the scale’s MCID.
Difficulty/Delays Accessing Care and PedsQL Scores
Having any difficulty/delays accessing care was significantly associated with lower baseline and follow-up PedsQL scores (Table 2). In adjusted analyses, the difference in baseline scores was 5.2 points (95% CI: −7.2, −3.2), which exceedes the scale’s MCID.
Interaction Between Social Disadvantage Markers, Difficulty/Delays Accessing Care, and Change in PedsQL Scores from Baseline to Follow-Up
While having =/>2 social disadvantage markers and difficulty/delays accessing care were each positively associated with changes in PedsQL scores from baseline to follow-up (Table 3), only patients with =/> 3 social disadvantage markers exceeded the PedsQL MCID. In stratified analyses, patients with a combination of social disadvantage makers and difficulties/delays accessing care had lower baseline PedsQL scores and greater change in PedsQL scores from baseline to follow-up compared with those without difficulties/delays accessing care (Figure). However, having any difficulty/delays accessing care did not significantly modify the relationship between social disadvantage and change in PedsQL scores, as none of the interaction terms were significant (Table 3, Model 3).
Sensitivity Analysis
Baseline, follow-up, and change in PedsQL scores were similar to our main analysis after performing multiple imputation for missing social disadvantage markers (Supplemental Table 1). Findings were also similar for patients with a diagnosis of asthma only; however, changes in PedsQL scores were greater in magnitude (Appendix Table 2).
DISCUSSION
This study examined the relationship between social disadvantage and child physical functioning before and after hospitalization for acute respiratory illness. Study findings indicated that patients with higher numbers of social disadvantage markers reported lower PedsQL scores before hospitalization; however, differences in PedsQL scores were not apparent after hospitalization. Patients who experienced difficulty/delays accessing care also reported lower PedsQL scores at baseline. This difference was still significant but did not exceed the PedsQL MCID threshold after hospitalization. Difficulty/delays accessing care appeared to be an additional social disadvantage marker; however, it did not modify the relationship between social disadvantage and improvement in physical functioning.
The study findings at baseline are consistent with prior studies demonstrating a negative association between social disadvantage markers and HRQoL and a cumulative effect based on the number of social disadvantages.3,4,7,8 This study adds to the existing literature by examining how this relationship changes after hospitalization. As evidenced by the lack of association between social disadvantage markers and follow-up PedsQL scores, our findings suggest that receipt of inpatient care improved perceptions of physical functioning to a greater extent for patients with more social disadvantage markers (especially patients with =/> 3 social disadvantage markers). There are several potential reasons for these findings.
One possibility is that caregivers and/or patients with more social disadvantage markers are more influenced by context when assessing physical functioning. This could lead to an underestimation of functioning when asked to recall baseline physical functioning at the time of acute illness and overestimation of functioning after recovery from an illness. This possibility is consistent with a form of response bias, extreme response tendencies, in which lower socioeconomic subgroups tend to choose the more extreme response options of a scale.28 In the absence of longitudinal assessments of HRQoL across the care continuum over time, disentangling whether these differences are due to response bias or representative of true changes in physical functioning remains challenging.
Given that disparities in physical functioning at baseline were consistent with prior evidence, another possibility is that hospitalization provided an opportunity to address gaps in access and quality that may have existed for patients with social disadvantage in the community setting. The 24/7 nature of hospital care, usually from a multidisciplinary team of providers, lends itself to opportunities to receive intensive education related to the current illness or to address other health concerns that parents or providers identify during a hospital stay. For example, consistent and repetitive asthma education may be more beneficial to patients and families with more social disadvantage markers. The fact that the association between social disadvantage markers and change in physical functioning scores were greater for patients with asthma supports this reasoning. Hospital care may also provide an opportunity to address other unmet medical needs or psychosocial needs by providing efficient access to subspecialists, social workers, or interpreters. Further research is needed to elucidate whether families received additional services in the hospital setting that were not available to them prior to hospitalization, such as consistent interpreter use, social work engagement, and subspecialty/community referrals. Further studies should also determine whether the provision of equitable medical and social support services is associated with improvements in HRQoL disparities. Additionally, studies should examine whether physical functioning improvements following hospitalization return to baseline levels after a longer period of time and, if so, how we might sustain these reductions in HRQoL disparities. Such studies may identify tangible targets and interventions to reduce disparities in HRQoL for these children.
This study highlights the importance of assessing for difficulty/delays accessing care in addition to social disadvantage markers, as this was also a significant predictor of lower child physical functioning. Differences in PedsQL scores between those who reported any versus no difficulty/delays accessing care were more pronounced at baseline compared with follow-up. A possible reason for these findings is that receiving hospital care may have addressed some access to care issues that were present in the outpatient setting, which resulted in improved perceptions of physical functioning. For example, hospital care may mitigate access to care barriers such as limited after-hours clinic appointments, language barriers, and lack of insurance, thus providing some patients with an alternative pathway to address their health concerns. Alternatively, hospital staff may assist families in scheduling follow-up appointments with the patient’s primary medical home after discharge, which potentially reduced some access to care barriers. The question is whether these disparities will widen once again after a longer follow-up period if families continue facing barriers to accessing needed care in the outpatient setting.
The results of the effect modification analysis demonstrated that the association between social disadvantage and change in PedsQL scores from baseline to follow-up was not significantly different based on a child’s ability to access care. In our stratified analysis, difficulty/delays accessing care added to baseline disparities at each social disadvantage level but did not alter how perceptions of physical functioning change over time. Therefore, physical functioning improvements may rely more heavily on the type of care received within the hospital setting as opposed to accessing care in the first place. However, future studies should examine whether access to high-quality care instead of simply measuring difficulty/delays in accessing care would lead to different results. Access to a comprehensive medical home may be a better measure to assess for effect modification because it measures features beyond access to care, such as continuity, comprehensiveness, communication, and coordination of outpatient care.29-31
If additional studies find evidence that the nature of hospital care, an intensive 24/7 care setting, differentially benefits patients with higher social disadvantage markers (particularly those with =/> 3 markers and chronic illness), this would support the need for systematic screening for social disadvantages or difficulty/delays accessing care in the inpatient setting. Systematic screening could help ensure all patients who may benefit from additional services, such as intensive, culturally tailored education or connections to food, housing, or financial services, will in fact receive them, which may lead to sustained reductions in health disparities.20 Further research into pairing validated screening tools with proven interventions is needed.32
This study has additional limitations aside from those noted above. First, we did not reassess perceived or actual access to care after hospitalization, which may have allowed for analyses to examine access to care as a mediator between social disadvantage and lower child physical functioning. Second, this study included only English- and Spanish-speaking patients and families. Patients with less commonly spoken languages may experience more difficulty accessing or navigating the health system, which may further impact access to care and HRQoL. Third, we had a considerable amount of missing social disadvantage marker data (mainly income); however, our sensitivity analyses did not result in significantly different or clinically meaningful differences in our findings. Insurance status is more feasible to obtain from administrative data and could serve as a proxy for income or as an additional social disadvantage marker in future studies. Finally, we could calculate illness severity only for patients with asthma based on the available data; therefore, we could not adequately control for illness severity across all conditions.
CONCLUSIONS
Social disadvantage was associated with lower child physical functioning before hospitalization, but differences were not apparent after hospitalization for children with acute respiratory illness. Caregiver-perceived difficulty/delays accessing care was found to be an additional predictor of lower physical functioning at baseline but did not significantly alter the association between social disadvantage and improvement in physical functioning over time. Further studies are needed to understand how hospital care may differentially impact child physical functioning for patients with higher social disadvantage makers in order to sustain improvements in HRQoL disparities.
Acknowledgments
The authors thank the following individuals of the Pediatric Respiratory Illness Measurement System (PRIMES) study team for their contributions to this work: Karen M. Wilson, New York, New York; Ricardo A. Quinonez, Houston, Texas; Joyee G. Vachani, Houston, Texas; and Amy Tyler, Aurora, Colorado. We would also like to thank the Pediatric Research in Inpatient Settings Network for facilitating this work.
1. Szilagyi PG, Schor EL. The health of children. Health Serv Res. 1998;33(4 Pt 2):1001-1039.
2. Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3(1):34. https://doi.org/10.1186/1477-7525-3-34.
3. von Rueden U, Gosch A, Rajmil L, Bisegger C, Ravens-Sieberer U. Socioeconomic determinants of health related quality of life in childhood and adolescence: results from a European study. J Epidemiol Community Health. 2006;60(2):130-135. https://doi.org/10.1136/jech.2005.039792.
4. Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137(3):642-650. https://doi.org/10.1378/chest.09-0345.
5. Flores G, Tomany-Korman SC, Corey CR, Freeman HE, Shapiro MF. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121(2):e286-98. https://doi.org/10.1542/peds.2007-1243.
6. Fedele DA, Molzon ES, Eddington AR, Hullmann SE, Mullins LL, Gillaspy SG. Perceived barriers to care in a pediatric medical home: the moderating role of caregiver minority status. Clin Pediatr (Phila). 2014;53(4):351-355. https://doi.org/10.1177/0009922813507994.
7. Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children’s health. Pediatrics. 2008;121(2):337-344. https://doi.org/10.1542/peds.2007-0447.
8. Bauman LJ, Silver EJ, Stein REK. Cumulative social disadvantage and child health. Pediatrics. 2006;117(4):1321-1328. https://doi.org/10.1542/peds.2005-1647.
9. Andrulis DP. Moving beyond the status quo in reducing racial and ethnic disparities in children’s health. Public Health Rep. 2005;120(4):370-377. https://doi.org/10.1177/003335490512000403.
10. Flores G, Lin H. Trends in racial/ethnic disparities in medical and oral health, access to care, and use of services in US children: has anything changed over the years? Int J Equity Health. 2013;12:10. https://doi.org/10.1186/1475-9276-12-10.
11. Seid M, Stevens GD, Varni JW. Parents’ perceptions of pediatric primary care quality: effects of race/ethnicity, language, and access. Health Serv Res. 2003;38(4):1009-1031. https://doi.org/10.1111/1475-6773.00160.
12. Seid M, Varni JW, Cummings L, Schonlau M. The impact of realized access to care on health-related quality of life: a two-year prospective cohort study of children in the California State Children’s Health Insurance Program. J Pediatr. 2006;149(3):354-361. https://doi.org/10.1016/j.jpeds.2006.04.024.
13. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. https://doi.xorg/10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2.
14. Simon AE, Chan KS, Forrest CB. Assessment of children’s health-related quality of life in the united states with a multidimensional index. Pediatrics. 2008;121(1):e118-e126. https://doi.org/10.1542/peds.2007-0480.
15. Cheng TL, Emmanuel MA, Levy DJ, Jenkins RR. Child health disparities: what can a clinician do? Pediatrics. 2015;136(5):961-968. https://doi.org/10.1542/peds.2014-4126.
16. Christakis DA, Mell L, Koepsell TD, Zimmerman FJ, Connell FA. Association of lower continuity of care with greater risk of emergency department use and hospitalization in children. Pediatrics. 2001;107(3):524-529. https://doi.org/10.1542/peds.107.3.524.
17. Lion KC, Raphael JL. Partnering health disparities research with quality improvement science in pediatrics. Pediatrics. 2015;135(2):354-361. https://doi.org/10.1542/peds.2014-2982.
18. Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14:S8-S17. https://doi.org/10.1097/01.PHH.0000338382.36695.42.
19. Beck AF, Cohen AJ, Colvin JD, et al. Perspectives from the Society for Pediatric Research: interventions targeting social needs in pediatric clinical care. Pediatr Res. 2018;84(1):10-21. https://doi.org/10.1038/s41390-018-0012-1.
20. Shah AN, Simmons J, Beck AF. Adding a vital sign: considering the utility of place-based measures in health care settings. Hosp Pediatr. 2018;8(2):112-114. https://doi.org/10.1542/hpeds.2017-0219.
21. Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
22. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. https://doi.org/10.1001/jamapediatrics.2014.1600.
23. Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and health-related quality of life after pediatric inpatient surgery. J Pain. 2015;16(12):1334-1341. https://doi.org/10.1016/j.jpain.2015.09.005.
24. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric respiratory illness measurement system (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242.
25. Child and Adolescent Health Measurement Initiative. National survey of children with special health care needs (NS-CSHCN), 2009-2010. Available at: http://childhealthdata.org/learn/NS-CSHCN/topics_questions. Accessed on September 20, 2018.
26. Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:1. https://doi.org/10.1186/1477-7525-5-1.
27. Buuren S van, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. https://doi.org/10.18637/jss.v045.i03.
28. Elliott MN, Haviland AM, Kanouse DE, Hambarsoomian K, Hays RD. Adjusting for subgroup differences in extreme response tendency in ratings of health care: impact on disparity estimates. Heal Serv Res. 2009;44(2 Pt 1):542-561. https://doi.org/10.1111/j.1475-6773.2008.00922.x.
29. Stevens GD, Vane C, Cousineau MR. Association of experiences of medical home quality with health-related quality of life and school engagement among Latino children in low-income families. Health Serv Res. 2011;46(6pt1):1822-1842. https://doi.org/10.1111/j.1475-6773.2011.01292.x.
30. Long WE, Bauchner H, Sege RD, Cabral HJ, Garg A. The value of the medical home for children without special health care needs. Pediatrics. 2012;129(1):87-98. https://doi.org/10.1542/peds.2011-1739.
31. Strickland BB, Jones JR, Ghandour RM, Kogan MD, Newacheck PW. The medical home: health care access and impact for children and youth in the United States. Pediatrics. 2011;127(4):604-611. https://doi.org/10.1542/peds.2009-3555.
32. Sokol R, Austin A, Chandler C, et al. Screening children for social determinants of health: a systematic review. Pediatrics. 2019;144(4):e20191622. https://doi.org/10.1542/peds.2019-1622.
Examining disparities in health-related quality of life (HRQoL) outcomes in children provides a unique patient-centered perspective on pediatric health services equity.1,2 Prior studies have demonstrated the relationship between minority race, low socioeconomic status, and lower maternal education with poorer HRQoL outcomes in children.3-6 Some studies have also shown a dose-response relationship between social disadvantage markers and poorer child health status.7,8 Furthermore, the associations between social disadvantage and poor access to care,9-11 and between poor access to care and lower HRQoL, are also well established.12-14
Examining HRQoL before and after hospitalization can further our understanding of how disparities in HRQoL may change once children engage with the medical system for an acute illness.15 Children requiring hospitalization constitute a useful population for examination of this question as they represent a group of children with variable social disadvantage markers and access to outpatient care.16 Although interventions to address social determinants of health for patients with social disadvantages have been associated with within-group improvements in HRQoL, none have assessed changes in disparities as an outcome.17 Furthermore, many of these studies were conducted in the outpatient setting,18,19 whereas hospitalization provides an additional point of care to address the social determinants of health for vulnerable families.20 Even for short hospitalizations, the 24/7 nature of hospital care provides the opportunity for frequent interactions with clinicians, nurses, and support staff to clarify illness-related questions, discuss other health concerns and unmet needs, and connect with social services or community resources. These opportunities may be particularly important for families with a higher number of social disadvantage markers and even more beneficial to those with difficulty accessing needed care from their primary medical home.
In this study, we focused on children with common respiratory illnesses (asthma, bronchiolitis, and pneumonia), which constitute the majority of childhood hospitalizations.21 Additionally, we only focused on the child’s physical functioning component of HRQoL because this component is most likely to improve after hospitalization for children with an acute respiratory illness.22 A prior study examining HRQoL before and after hospitalization demonstrated that most children return to and/or surpass their baseline physical functioning by 1 month after hospital discharge.23
Our primary objective was to examine associations between several markers of social disadvantage, access to care, and child physical functioning before and after hospitalization for acute respiratory illness. Second, we aimed to understand if access to care (defined as perceived difficulty/delays getting care) acts as an independent predictor of improvement in physical functioning from baseline to follow-up and/or if it modifies the relationship between social disadvantage and improvement in physical functioning (Appendix Figure).
METHODS
Study Design and Population
This study was nested within a multicenter, prospective cohort study of children who were hospitalized for asthma, bronchiolitis, or pneumonia between July 2014 and June 2016 at one of five children’s hospitals in the Pediatric Research in Inpatient Settings Network.24
We approached families for study participation within 72 hours of admission to the hospital using a standard protocol. Patients and their caregivers were eligible to participate in the study if the patient was 2 weeks to 16 years old and if the primary caregiver’s preferred language for medical communication was either English or Spanish. Patients with chronic medical conditions (except asthma), with moderate to severe developmental delay, with a history of prematurity <32 weeks, or who received care in the intensive care unit were excluded. Patients could only participate in the study once.
The study team set out to enroll an even number of patients across all three conditions. If a patient’s discharge diagnosis differed from their admission diagnosis (eg, from bronchiolitis to pneumonia), discharge diagnosis was used for condition group assignment. If the discharge diagnosis was not one of these three respiratory conditions, we excluded the patient from further analysis.
Data Collection
We collected data using two surveys. The first survey was administered within 72 hours of admission. This survey asked questions related to (1) caregiver-reported markers of social disadvantage, (2) caregiver perceptions of access to care, and (3) caregiver- and patient-reported assessments of physical functioning. The second survey was administered within 2 to 8 weeks after the patient’s discharge and included a second assessment of physical functioning.
Social Disadvantage
Patients were considered to have a marker of social disadvantage if their caregiver reported (1) being of non-White race and/or Hispanic ethnicity, (2) primarily speaking a language other than English at home and not speaking English very well (ie, limited English proficiency), (3) attaining at most a high school or equivalent degree, or (4) having a =/<$30,000 annual household income.
Access to Care
We used the following survey item from the 2009-2010 National Survey of Children with Special Health Care Needs25 to measure caregiver perceptions of access to care: “In the last six months, did you have any difficulties or delays getting care for your child because there were waiting lists, backlogs, or other problems getting an appointment?” We narrowed the original assessment time frame from 12 months to 6 months to provide a more proximal assessment of access in relation to the hospitalization.
Child Physical Functioning
We assessed child physical functioning using the physical functioning domain of the Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales and PedsQL Infant Scales, which have been validated for use in the inpatient setting.22 Caregivers completed one of these scales based on their child’s age. Assenting patients 8 to 16 years old completed the self-report PedsQL 4.0 Generic Core Scales instrument. When completing the first PedsQL survey, caregivers and patients reflected on the previous month before their child (or they) became ill to obtain a baseline physical functioning assessment.23 When completing the second PedsQL survey, caregivers and patients reflected on the past 7 days to obtain a follow-up assessment.
All study procedures were approved by the Western Institutional Review Board (IRB) or the participating hospitals’ IRB.
Statistical Analysis
Patients with no missing data for all four social disadvantage markers were categorized based on the number of markers they reported: none, one, two, or three or more markers. We combined patients with three and four social disadvantage markers into one group to maximize power for the analyses. We dichotomized the access to care variable and coded response options as “no difficulty/delays accessing care” if the caregiver chose “Never” and “any difficulty/delays accessing care” if they chose “Sometimes/Usually/Always.”
For each patient–caregiver dyad, PedsQL items were scored using a standard method in which higher scores reflected better functioning.22 A single set of PedsQL scores was used for each patient–caregiver dyad. We used self-reported patient scores if the patient completed the PedsQL instrument; otherwise, we used proxy-reported caregiver scores. Intraclass correlations between child self-report and parent proxy-report demonstrate moderate to good agreement above age 8 years.26 We computed a change in the physical functioning score by subtracting the baseline score from the follow-up score. The minimal clinically important difference (MCID) for the PedsQL instrument is 4.5 points, which we used to identify clinically meaningful differences.13
Analysis of variance models were constructed to test for differences in mean baseline and follow-up PedsQL scores (dependent variable) between the following independent variables: (1) social disadvantage groups and (2) those who reported having any difficulty/delays accessing care compared with those who did not. Only patient–caregiver dyads with both baseline and follow-up assessments were included in these analyses. Mixed-effects linear regression models were constructed to identify clinically meaningful differences in PedsQL scores between groups (MCID =/> 4.5) with adjustment for patient age, gender, respiratory condition, days between surveys, and hospital site as fixed effects. Site-specific random effects were included to account for within-hospital clustering. A similarly adjusted mixed-effects linear regression model was constructed to examine whether having any difficulty/delays accessing care modified the association between social disadvantage and PedsQL change scores (eg, an improvement from baseline to follow-up).
Because 17% of respondents had missing data for at least one social disadvantage marker, sensitivity analyses were conducted using multiple imputation to account for missing social disadvantage markers using chained equations.27 Sensitivity analyses were also conducted to adjust for severity of illness using vital sign data within the first 24 hours, which could only be validly captured on patients with asthma within our dataset. By restricting this latter analysis to patients with asthma, we were able to examine the relationships of interest in a population with chronic disease.
RESULTS
The study sample included 1,860 patients, of which 1,325 had both baseline and follow-up PedsQL data (71%). Descriptive statistics were similar between those who completed the baseline and follow-up surveys (Table 1).
Twenty-two percent of patients had >/=3 social disadvantages and 30% of caregivers reported having any difficulty/delays accessing care. The mean follow-up PedsQL score was higher than the baseline score (90.4 vs 82.5; Table 1).
Social Disadvantage Markers and PedsQL Scores
The number of social disadvantage markers was inversely related to mean baseline PedsQL scores, but there was no difference in mean follow-up PedsQL scores between social disadvantage groups (Table 2). In adjusted analyses, the mean baseline PedsQL score was −6.1 points (95% CI: −8.7, −3.5) lower for patients with >/= 3 social disadvantage markers compared with patients with no social disadvantage markers, which exceeded the scale’s MCID.
Difficulty/Delays Accessing Care and PedsQL Scores
Having any difficulty/delays accessing care was significantly associated with lower baseline and follow-up PedsQL scores (Table 2). In adjusted analyses, the difference in baseline scores was 5.2 points (95% CI: −7.2, −3.2), which exceedes the scale’s MCID.
Interaction Between Social Disadvantage Markers, Difficulty/Delays Accessing Care, and Change in PedsQL Scores from Baseline to Follow-Up
While having =/>2 social disadvantage markers and difficulty/delays accessing care were each positively associated with changes in PedsQL scores from baseline to follow-up (Table 3), only patients with =/> 3 social disadvantage markers exceeded the PedsQL MCID. In stratified analyses, patients with a combination of social disadvantage makers and difficulties/delays accessing care had lower baseline PedsQL scores and greater change in PedsQL scores from baseline to follow-up compared with those without difficulties/delays accessing care (Figure). However, having any difficulty/delays accessing care did not significantly modify the relationship between social disadvantage and change in PedsQL scores, as none of the interaction terms were significant (Table 3, Model 3).
Sensitivity Analysis
Baseline, follow-up, and change in PedsQL scores were similar to our main analysis after performing multiple imputation for missing social disadvantage markers (Supplemental Table 1). Findings were also similar for patients with a diagnosis of asthma only; however, changes in PedsQL scores were greater in magnitude (Appendix Table 2).
DISCUSSION
This study examined the relationship between social disadvantage and child physical functioning before and after hospitalization for acute respiratory illness. Study findings indicated that patients with higher numbers of social disadvantage markers reported lower PedsQL scores before hospitalization; however, differences in PedsQL scores were not apparent after hospitalization. Patients who experienced difficulty/delays accessing care also reported lower PedsQL scores at baseline. This difference was still significant but did not exceed the PedsQL MCID threshold after hospitalization. Difficulty/delays accessing care appeared to be an additional social disadvantage marker; however, it did not modify the relationship between social disadvantage and improvement in physical functioning.
The study findings at baseline are consistent with prior studies demonstrating a negative association between social disadvantage markers and HRQoL and a cumulative effect based on the number of social disadvantages.3,4,7,8 This study adds to the existing literature by examining how this relationship changes after hospitalization. As evidenced by the lack of association between social disadvantage markers and follow-up PedsQL scores, our findings suggest that receipt of inpatient care improved perceptions of physical functioning to a greater extent for patients with more social disadvantage markers (especially patients with =/> 3 social disadvantage markers). There are several potential reasons for these findings.
One possibility is that caregivers and/or patients with more social disadvantage markers are more influenced by context when assessing physical functioning. This could lead to an underestimation of functioning when asked to recall baseline physical functioning at the time of acute illness and overestimation of functioning after recovery from an illness. This possibility is consistent with a form of response bias, extreme response tendencies, in which lower socioeconomic subgroups tend to choose the more extreme response options of a scale.28 In the absence of longitudinal assessments of HRQoL across the care continuum over time, disentangling whether these differences are due to response bias or representative of true changes in physical functioning remains challenging.
Given that disparities in physical functioning at baseline were consistent with prior evidence, another possibility is that hospitalization provided an opportunity to address gaps in access and quality that may have existed for patients with social disadvantage in the community setting. The 24/7 nature of hospital care, usually from a multidisciplinary team of providers, lends itself to opportunities to receive intensive education related to the current illness or to address other health concerns that parents or providers identify during a hospital stay. For example, consistent and repetitive asthma education may be more beneficial to patients and families with more social disadvantage markers. The fact that the association between social disadvantage markers and change in physical functioning scores were greater for patients with asthma supports this reasoning. Hospital care may also provide an opportunity to address other unmet medical needs or psychosocial needs by providing efficient access to subspecialists, social workers, or interpreters. Further research is needed to elucidate whether families received additional services in the hospital setting that were not available to them prior to hospitalization, such as consistent interpreter use, social work engagement, and subspecialty/community referrals. Further studies should also determine whether the provision of equitable medical and social support services is associated with improvements in HRQoL disparities. Additionally, studies should examine whether physical functioning improvements following hospitalization return to baseline levels after a longer period of time and, if so, how we might sustain these reductions in HRQoL disparities. Such studies may identify tangible targets and interventions to reduce disparities in HRQoL for these children.
This study highlights the importance of assessing for difficulty/delays accessing care in addition to social disadvantage markers, as this was also a significant predictor of lower child physical functioning. Differences in PedsQL scores between those who reported any versus no difficulty/delays accessing care were more pronounced at baseline compared with follow-up. A possible reason for these findings is that receiving hospital care may have addressed some access to care issues that were present in the outpatient setting, which resulted in improved perceptions of physical functioning. For example, hospital care may mitigate access to care barriers such as limited after-hours clinic appointments, language barriers, and lack of insurance, thus providing some patients with an alternative pathway to address their health concerns. Alternatively, hospital staff may assist families in scheduling follow-up appointments with the patient’s primary medical home after discharge, which potentially reduced some access to care barriers. The question is whether these disparities will widen once again after a longer follow-up period if families continue facing barriers to accessing needed care in the outpatient setting.
The results of the effect modification analysis demonstrated that the association between social disadvantage and change in PedsQL scores from baseline to follow-up was not significantly different based on a child’s ability to access care. In our stratified analysis, difficulty/delays accessing care added to baseline disparities at each social disadvantage level but did not alter how perceptions of physical functioning change over time. Therefore, physical functioning improvements may rely more heavily on the type of care received within the hospital setting as opposed to accessing care in the first place. However, future studies should examine whether access to high-quality care instead of simply measuring difficulty/delays in accessing care would lead to different results. Access to a comprehensive medical home may be a better measure to assess for effect modification because it measures features beyond access to care, such as continuity, comprehensiveness, communication, and coordination of outpatient care.29-31
If additional studies find evidence that the nature of hospital care, an intensive 24/7 care setting, differentially benefits patients with higher social disadvantage markers (particularly those with =/> 3 markers and chronic illness), this would support the need for systematic screening for social disadvantages or difficulty/delays accessing care in the inpatient setting. Systematic screening could help ensure all patients who may benefit from additional services, such as intensive, culturally tailored education or connections to food, housing, or financial services, will in fact receive them, which may lead to sustained reductions in health disparities.20 Further research into pairing validated screening tools with proven interventions is needed.32
This study has additional limitations aside from those noted above. First, we did not reassess perceived or actual access to care after hospitalization, which may have allowed for analyses to examine access to care as a mediator between social disadvantage and lower child physical functioning. Second, this study included only English- and Spanish-speaking patients and families. Patients with less commonly spoken languages may experience more difficulty accessing or navigating the health system, which may further impact access to care and HRQoL. Third, we had a considerable amount of missing social disadvantage marker data (mainly income); however, our sensitivity analyses did not result in significantly different or clinically meaningful differences in our findings. Insurance status is more feasible to obtain from administrative data and could serve as a proxy for income or as an additional social disadvantage marker in future studies. Finally, we could calculate illness severity only for patients with asthma based on the available data; therefore, we could not adequately control for illness severity across all conditions.
CONCLUSIONS
Social disadvantage was associated with lower child physical functioning before hospitalization, but differences were not apparent after hospitalization for children with acute respiratory illness. Caregiver-perceived difficulty/delays accessing care was found to be an additional predictor of lower physical functioning at baseline but did not significantly alter the association between social disadvantage and improvement in physical functioning over time. Further studies are needed to understand how hospital care may differentially impact child physical functioning for patients with higher social disadvantage makers in order to sustain improvements in HRQoL disparities.
Acknowledgments
The authors thank the following individuals of the Pediatric Respiratory Illness Measurement System (PRIMES) study team for their contributions to this work: Karen M. Wilson, New York, New York; Ricardo A. Quinonez, Houston, Texas; Joyee G. Vachani, Houston, Texas; and Amy Tyler, Aurora, Colorado. We would also like to thank the Pediatric Research in Inpatient Settings Network for facilitating this work.
Examining disparities in health-related quality of life (HRQoL) outcomes in children provides a unique patient-centered perspective on pediatric health services equity.1,2 Prior studies have demonstrated the relationship between minority race, low socioeconomic status, and lower maternal education with poorer HRQoL outcomes in children.3-6 Some studies have also shown a dose-response relationship between social disadvantage markers and poorer child health status.7,8 Furthermore, the associations between social disadvantage and poor access to care,9-11 and between poor access to care and lower HRQoL, are also well established.12-14
Examining HRQoL before and after hospitalization can further our understanding of how disparities in HRQoL may change once children engage with the medical system for an acute illness.15 Children requiring hospitalization constitute a useful population for examination of this question as they represent a group of children with variable social disadvantage markers and access to outpatient care.16 Although interventions to address social determinants of health for patients with social disadvantages have been associated with within-group improvements in HRQoL, none have assessed changes in disparities as an outcome.17 Furthermore, many of these studies were conducted in the outpatient setting,18,19 whereas hospitalization provides an additional point of care to address the social determinants of health for vulnerable families.20 Even for short hospitalizations, the 24/7 nature of hospital care provides the opportunity for frequent interactions with clinicians, nurses, and support staff to clarify illness-related questions, discuss other health concerns and unmet needs, and connect with social services or community resources. These opportunities may be particularly important for families with a higher number of social disadvantage markers and even more beneficial to those with difficulty accessing needed care from their primary medical home.
In this study, we focused on children with common respiratory illnesses (asthma, bronchiolitis, and pneumonia), which constitute the majority of childhood hospitalizations.21 Additionally, we only focused on the child’s physical functioning component of HRQoL because this component is most likely to improve after hospitalization for children with an acute respiratory illness.22 A prior study examining HRQoL before and after hospitalization demonstrated that most children return to and/or surpass their baseline physical functioning by 1 month after hospital discharge.23
Our primary objective was to examine associations between several markers of social disadvantage, access to care, and child physical functioning before and after hospitalization for acute respiratory illness. Second, we aimed to understand if access to care (defined as perceived difficulty/delays getting care) acts as an independent predictor of improvement in physical functioning from baseline to follow-up and/or if it modifies the relationship between social disadvantage and improvement in physical functioning (Appendix Figure).
METHODS
Study Design and Population
This study was nested within a multicenter, prospective cohort study of children who were hospitalized for asthma, bronchiolitis, or pneumonia between July 2014 and June 2016 at one of five children’s hospitals in the Pediatric Research in Inpatient Settings Network.24
We approached families for study participation within 72 hours of admission to the hospital using a standard protocol. Patients and their caregivers were eligible to participate in the study if the patient was 2 weeks to 16 years old and if the primary caregiver’s preferred language for medical communication was either English or Spanish. Patients with chronic medical conditions (except asthma), with moderate to severe developmental delay, with a history of prematurity <32 weeks, or who received care in the intensive care unit were excluded. Patients could only participate in the study once.
The study team set out to enroll an even number of patients across all three conditions. If a patient’s discharge diagnosis differed from their admission diagnosis (eg, from bronchiolitis to pneumonia), discharge diagnosis was used for condition group assignment. If the discharge diagnosis was not one of these three respiratory conditions, we excluded the patient from further analysis.
Data Collection
We collected data using two surveys. The first survey was administered within 72 hours of admission. This survey asked questions related to (1) caregiver-reported markers of social disadvantage, (2) caregiver perceptions of access to care, and (3) caregiver- and patient-reported assessments of physical functioning. The second survey was administered within 2 to 8 weeks after the patient’s discharge and included a second assessment of physical functioning.
Social Disadvantage
Patients were considered to have a marker of social disadvantage if their caregiver reported (1) being of non-White race and/or Hispanic ethnicity, (2) primarily speaking a language other than English at home and not speaking English very well (ie, limited English proficiency), (3) attaining at most a high school or equivalent degree, or (4) having a =/<$30,000 annual household income.
Access to Care
We used the following survey item from the 2009-2010 National Survey of Children with Special Health Care Needs25 to measure caregiver perceptions of access to care: “In the last six months, did you have any difficulties or delays getting care for your child because there were waiting lists, backlogs, or other problems getting an appointment?” We narrowed the original assessment time frame from 12 months to 6 months to provide a more proximal assessment of access in relation to the hospitalization.
Child Physical Functioning
We assessed child physical functioning using the physical functioning domain of the Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales and PedsQL Infant Scales, which have been validated for use in the inpatient setting.22 Caregivers completed one of these scales based on their child’s age. Assenting patients 8 to 16 years old completed the self-report PedsQL 4.0 Generic Core Scales instrument. When completing the first PedsQL survey, caregivers and patients reflected on the previous month before their child (or they) became ill to obtain a baseline physical functioning assessment.23 When completing the second PedsQL survey, caregivers and patients reflected on the past 7 days to obtain a follow-up assessment.
All study procedures were approved by the Western Institutional Review Board (IRB) or the participating hospitals’ IRB.
Statistical Analysis
Patients with no missing data for all four social disadvantage markers were categorized based on the number of markers they reported: none, one, two, or three or more markers. We combined patients with three and four social disadvantage markers into one group to maximize power for the analyses. We dichotomized the access to care variable and coded response options as “no difficulty/delays accessing care” if the caregiver chose “Never” and “any difficulty/delays accessing care” if they chose “Sometimes/Usually/Always.”
For each patient–caregiver dyad, PedsQL items were scored using a standard method in which higher scores reflected better functioning.22 A single set of PedsQL scores was used for each patient–caregiver dyad. We used self-reported patient scores if the patient completed the PedsQL instrument; otherwise, we used proxy-reported caregiver scores. Intraclass correlations between child self-report and parent proxy-report demonstrate moderate to good agreement above age 8 years.26 We computed a change in the physical functioning score by subtracting the baseline score from the follow-up score. The minimal clinically important difference (MCID) for the PedsQL instrument is 4.5 points, which we used to identify clinically meaningful differences.13
Analysis of variance models were constructed to test for differences in mean baseline and follow-up PedsQL scores (dependent variable) between the following independent variables: (1) social disadvantage groups and (2) those who reported having any difficulty/delays accessing care compared with those who did not. Only patient–caregiver dyads with both baseline and follow-up assessments were included in these analyses. Mixed-effects linear regression models were constructed to identify clinically meaningful differences in PedsQL scores between groups (MCID =/> 4.5) with adjustment for patient age, gender, respiratory condition, days between surveys, and hospital site as fixed effects. Site-specific random effects were included to account for within-hospital clustering. A similarly adjusted mixed-effects linear regression model was constructed to examine whether having any difficulty/delays accessing care modified the association between social disadvantage and PedsQL change scores (eg, an improvement from baseline to follow-up).
Because 17% of respondents had missing data for at least one social disadvantage marker, sensitivity analyses were conducted using multiple imputation to account for missing social disadvantage markers using chained equations.27 Sensitivity analyses were also conducted to adjust for severity of illness using vital sign data within the first 24 hours, which could only be validly captured on patients with asthma within our dataset. By restricting this latter analysis to patients with asthma, we were able to examine the relationships of interest in a population with chronic disease.
RESULTS
The study sample included 1,860 patients, of which 1,325 had both baseline and follow-up PedsQL data (71%). Descriptive statistics were similar between those who completed the baseline and follow-up surveys (Table 1).
Twenty-two percent of patients had >/=3 social disadvantages and 30% of caregivers reported having any difficulty/delays accessing care. The mean follow-up PedsQL score was higher than the baseline score (90.4 vs 82.5; Table 1).
Social Disadvantage Markers and PedsQL Scores
The number of social disadvantage markers was inversely related to mean baseline PedsQL scores, but there was no difference in mean follow-up PedsQL scores between social disadvantage groups (Table 2). In adjusted analyses, the mean baseline PedsQL score was −6.1 points (95% CI: −8.7, −3.5) lower for patients with >/= 3 social disadvantage markers compared with patients with no social disadvantage markers, which exceeded the scale’s MCID.
Difficulty/Delays Accessing Care and PedsQL Scores
Having any difficulty/delays accessing care was significantly associated with lower baseline and follow-up PedsQL scores (Table 2). In adjusted analyses, the difference in baseline scores was 5.2 points (95% CI: −7.2, −3.2), which exceedes the scale’s MCID.
Interaction Between Social Disadvantage Markers, Difficulty/Delays Accessing Care, and Change in PedsQL Scores from Baseline to Follow-Up
While having =/>2 social disadvantage markers and difficulty/delays accessing care were each positively associated with changes in PedsQL scores from baseline to follow-up (Table 3), only patients with =/> 3 social disadvantage markers exceeded the PedsQL MCID. In stratified analyses, patients with a combination of social disadvantage makers and difficulties/delays accessing care had lower baseline PedsQL scores and greater change in PedsQL scores from baseline to follow-up compared with those without difficulties/delays accessing care (Figure). However, having any difficulty/delays accessing care did not significantly modify the relationship between social disadvantage and change in PedsQL scores, as none of the interaction terms were significant (Table 3, Model 3).
Sensitivity Analysis
Baseline, follow-up, and change in PedsQL scores were similar to our main analysis after performing multiple imputation for missing social disadvantage markers (Supplemental Table 1). Findings were also similar for patients with a diagnosis of asthma only; however, changes in PedsQL scores were greater in magnitude (Appendix Table 2).
DISCUSSION
This study examined the relationship between social disadvantage and child physical functioning before and after hospitalization for acute respiratory illness. Study findings indicated that patients with higher numbers of social disadvantage markers reported lower PedsQL scores before hospitalization; however, differences in PedsQL scores were not apparent after hospitalization. Patients who experienced difficulty/delays accessing care also reported lower PedsQL scores at baseline. This difference was still significant but did not exceed the PedsQL MCID threshold after hospitalization. Difficulty/delays accessing care appeared to be an additional social disadvantage marker; however, it did not modify the relationship between social disadvantage and improvement in physical functioning.
The study findings at baseline are consistent with prior studies demonstrating a negative association between social disadvantage markers and HRQoL and a cumulative effect based on the number of social disadvantages.3,4,7,8 This study adds to the existing literature by examining how this relationship changes after hospitalization. As evidenced by the lack of association between social disadvantage markers and follow-up PedsQL scores, our findings suggest that receipt of inpatient care improved perceptions of physical functioning to a greater extent for patients with more social disadvantage markers (especially patients with =/> 3 social disadvantage markers). There are several potential reasons for these findings.
One possibility is that caregivers and/or patients with more social disadvantage markers are more influenced by context when assessing physical functioning. This could lead to an underestimation of functioning when asked to recall baseline physical functioning at the time of acute illness and overestimation of functioning after recovery from an illness. This possibility is consistent with a form of response bias, extreme response tendencies, in which lower socioeconomic subgroups tend to choose the more extreme response options of a scale.28 In the absence of longitudinal assessments of HRQoL across the care continuum over time, disentangling whether these differences are due to response bias or representative of true changes in physical functioning remains challenging.
Given that disparities in physical functioning at baseline were consistent with prior evidence, another possibility is that hospitalization provided an opportunity to address gaps in access and quality that may have existed for patients with social disadvantage in the community setting. The 24/7 nature of hospital care, usually from a multidisciplinary team of providers, lends itself to opportunities to receive intensive education related to the current illness or to address other health concerns that parents or providers identify during a hospital stay. For example, consistent and repetitive asthma education may be more beneficial to patients and families with more social disadvantage markers. The fact that the association between social disadvantage markers and change in physical functioning scores were greater for patients with asthma supports this reasoning. Hospital care may also provide an opportunity to address other unmet medical needs or psychosocial needs by providing efficient access to subspecialists, social workers, or interpreters. Further research is needed to elucidate whether families received additional services in the hospital setting that were not available to them prior to hospitalization, such as consistent interpreter use, social work engagement, and subspecialty/community referrals. Further studies should also determine whether the provision of equitable medical and social support services is associated with improvements in HRQoL disparities. Additionally, studies should examine whether physical functioning improvements following hospitalization return to baseline levels after a longer period of time and, if so, how we might sustain these reductions in HRQoL disparities. Such studies may identify tangible targets and interventions to reduce disparities in HRQoL for these children.
This study highlights the importance of assessing for difficulty/delays accessing care in addition to social disadvantage markers, as this was also a significant predictor of lower child physical functioning. Differences in PedsQL scores between those who reported any versus no difficulty/delays accessing care were more pronounced at baseline compared with follow-up. A possible reason for these findings is that receiving hospital care may have addressed some access to care issues that were present in the outpatient setting, which resulted in improved perceptions of physical functioning. For example, hospital care may mitigate access to care barriers such as limited after-hours clinic appointments, language barriers, and lack of insurance, thus providing some patients with an alternative pathway to address their health concerns. Alternatively, hospital staff may assist families in scheduling follow-up appointments with the patient’s primary medical home after discharge, which potentially reduced some access to care barriers. The question is whether these disparities will widen once again after a longer follow-up period if families continue facing barriers to accessing needed care in the outpatient setting.
The results of the effect modification analysis demonstrated that the association between social disadvantage and change in PedsQL scores from baseline to follow-up was not significantly different based on a child’s ability to access care. In our stratified analysis, difficulty/delays accessing care added to baseline disparities at each social disadvantage level but did not alter how perceptions of physical functioning change over time. Therefore, physical functioning improvements may rely more heavily on the type of care received within the hospital setting as opposed to accessing care in the first place. However, future studies should examine whether access to high-quality care instead of simply measuring difficulty/delays in accessing care would lead to different results. Access to a comprehensive medical home may be a better measure to assess for effect modification because it measures features beyond access to care, such as continuity, comprehensiveness, communication, and coordination of outpatient care.29-31
If additional studies find evidence that the nature of hospital care, an intensive 24/7 care setting, differentially benefits patients with higher social disadvantage markers (particularly those with =/> 3 markers and chronic illness), this would support the need for systematic screening for social disadvantages or difficulty/delays accessing care in the inpatient setting. Systematic screening could help ensure all patients who may benefit from additional services, such as intensive, culturally tailored education or connections to food, housing, or financial services, will in fact receive them, which may lead to sustained reductions in health disparities.20 Further research into pairing validated screening tools with proven interventions is needed.32
This study has additional limitations aside from those noted above. First, we did not reassess perceived or actual access to care after hospitalization, which may have allowed for analyses to examine access to care as a mediator between social disadvantage and lower child physical functioning. Second, this study included only English- and Spanish-speaking patients and families. Patients with less commonly spoken languages may experience more difficulty accessing or navigating the health system, which may further impact access to care and HRQoL. Third, we had a considerable amount of missing social disadvantage marker data (mainly income); however, our sensitivity analyses did not result in significantly different or clinically meaningful differences in our findings. Insurance status is more feasible to obtain from administrative data and could serve as a proxy for income or as an additional social disadvantage marker in future studies. Finally, we could calculate illness severity only for patients with asthma based on the available data; therefore, we could not adequately control for illness severity across all conditions.
CONCLUSIONS
Social disadvantage was associated with lower child physical functioning before hospitalization, but differences were not apparent after hospitalization for children with acute respiratory illness. Caregiver-perceived difficulty/delays accessing care was found to be an additional predictor of lower physical functioning at baseline but did not significantly alter the association between social disadvantage and improvement in physical functioning over time. Further studies are needed to understand how hospital care may differentially impact child physical functioning for patients with higher social disadvantage makers in order to sustain improvements in HRQoL disparities.
Acknowledgments
The authors thank the following individuals of the Pediatric Respiratory Illness Measurement System (PRIMES) study team for their contributions to this work: Karen M. Wilson, New York, New York; Ricardo A. Quinonez, Houston, Texas; Joyee G. Vachani, Houston, Texas; and Amy Tyler, Aurora, Colorado. We would also like to thank the Pediatric Research in Inpatient Settings Network for facilitating this work.
1. Szilagyi PG, Schor EL. The health of children. Health Serv Res. 1998;33(4 Pt 2):1001-1039.
2. Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3(1):34. https://doi.org/10.1186/1477-7525-3-34.
3. von Rueden U, Gosch A, Rajmil L, Bisegger C, Ravens-Sieberer U. Socioeconomic determinants of health related quality of life in childhood and adolescence: results from a European study. J Epidemiol Community Health. 2006;60(2):130-135. https://doi.org/10.1136/jech.2005.039792.
4. Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137(3):642-650. https://doi.org/10.1378/chest.09-0345.
5. Flores G, Tomany-Korman SC, Corey CR, Freeman HE, Shapiro MF. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121(2):e286-98. https://doi.org/10.1542/peds.2007-1243.
6. Fedele DA, Molzon ES, Eddington AR, Hullmann SE, Mullins LL, Gillaspy SG. Perceived barriers to care in a pediatric medical home: the moderating role of caregiver minority status. Clin Pediatr (Phila). 2014;53(4):351-355. https://doi.org/10.1177/0009922813507994.
7. Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children’s health. Pediatrics. 2008;121(2):337-344. https://doi.org/10.1542/peds.2007-0447.
8. Bauman LJ, Silver EJ, Stein REK. Cumulative social disadvantage and child health. Pediatrics. 2006;117(4):1321-1328. https://doi.org/10.1542/peds.2005-1647.
9. Andrulis DP. Moving beyond the status quo in reducing racial and ethnic disparities in children’s health. Public Health Rep. 2005;120(4):370-377. https://doi.org/10.1177/003335490512000403.
10. Flores G, Lin H. Trends in racial/ethnic disparities in medical and oral health, access to care, and use of services in US children: has anything changed over the years? Int J Equity Health. 2013;12:10. https://doi.org/10.1186/1475-9276-12-10.
11. Seid M, Stevens GD, Varni JW. Parents’ perceptions of pediatric primary care quality: effects of race/ethnicity, language, and access. Health Serv Res. 2003;38(4):1009-1031. https://doi.org/10.1111/1475-6773.00160.
12. Seid M, Varni JW, Cummings L, Schonlau M. The impact of realized access to care on health-related quality of life: a two-year prospective cohort study of children in the California State Children’s Health Insurance Program. J Pediatr. 2006;149(3):354-361. https://doi.org/10.1016/j.jpeds.2006.04.024.
13. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. https://doi.xorg/10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2.
14. Simon AE, Chan KS, Forrest CB. Assessment of children’s health-related quality of life in the united states with a multidimensional index. Pediatrics. 2008;121(1):e118-e126. https://doi.org/10.1542/peds.2007-0480.
15. Cheng TL, Emmanuel MA, Levy DJ, Jenkins RR. Child health disparities: what can a clinician do? Pediatrics. 2015;136(5):961-968. https://doi.org/10.1542/peds.2014-4126.
16. Christakis DA, Mell L, Koepsell TD, Zimmerman FJ, Connell FA. Association of lower continuity of care with greater risk of emergency department use and hospitalization in children. Pediatrics. 2001;107(3):524-529. https://doi.org/10.1542/peds.107.3.524.
17. Lion KC, Raphael JL. Partnering health disparities research with quality improvement science in pediatrics. Pediatrics. 2015;135(2):354-361. https://doi.org/10.1542/peds.2014-2982.
18. Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14:S8-S17. https://doi.org/10.1097/01.PHH.0000338382.36695.42.
19. Beck AF, Cohen AJ, Colvin JD, et al. Perspectives from the Society for Pediatric Research: interventions targeting social needs in pediatric clinical care. Pediatr Res. 2018;84(1):10-21. https://doi.org/10.1038/s41390-018-0012-1.
20. Shah AN, Simmons J, Beck AF. Adding a vital sign: considering the utility of place-based measures in health care settings. Hosp Pediatr. 2018;8(2):112-114. https://doi.org/10.1542/hpeds.2017-0219.
21. Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
22. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. https://doi.org/10.1001/jamapediatrics.2014.1600.
23. Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and health-related quality of life after pediatric inpatient surgery. J Pain. 2015;16(12):1334-1341. https://doi.org/10.1016/j.jpain.2015.09.005.
24. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric respiratory illness measurement system (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242.
25. Child and Adolescent Health Measurement Initiative. National survey of children with special health care needs (NS-CSHCN), 2009-2010. Available at: http://childhealthdata.org/learn/NS-CSHCN/topics_questions. Accessed on September 20, 2018.
26. Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:1. https://doi.org/10.1186/1477-7525-5-1.
27. Buuren S van, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. https://doi.org/10.18637/jss.v045.i03.
28. Elliott MN, Haviland AM, Kanouse DE, Hambarsoomian K, Hays RD. Adjusting for subgroup differences in extreme response tendency in ratings of health care: impact on disparity estimates. Heal Serv Res. 2009;44(2 Pt 1):542-561. https://doi.org/10.1111/j.1475-6773.2008.00922.x.
29. Stevens GD, Vane C, Cousineau MR. Association of experiences of medical home quality with health-related quality of life and school engagement among Latino children in low-income families. Health Serv Res. 2011;46(6pt1):1822-1842. https://doi.org/10.1111/j.1475-6773.2011.01292.x.
30. Long WE, Bauchner H, Sege RD, Cabral HJ, Garg A. The value of the medical home for children without special health care needs. Pediatrics. 2012;129(1):87-98. https://doi.org/10.1542/peds.2011-1739.
31. Strickland BB, Jones JR, Ghandour RM, Kogan MD, Newacheck PW. The medical home: health care access and impact for children and youth in the United States. Pediatrics. 2011;127(4):604-611. https://doi.org/10.1542/peds.2009-3555.
32. Sokol R, Austin A, Chandler C, et al. Screening children for social determinants of health: a systematic review. Pediatrics. 2019;144(4):e20191622. https://doi.org/10.1542/peds.2019-1622.
1. Szilagyi PG, Schor EL. The health of children. Health Serv Res. 1998;33(4 Pt 2):1001-1039.
2. Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3(1):34. https://doi.org/10.1186/1477-7525-3-34.
3. von Rueden U, Gosch A, Rajmil L, Bisegger C, Ravens-Sieberer U. Socioeconomic determinants of health related quality of life in childhood and adolescence: results from a European study. J Epidemiol Community Health. 2006;60(2):130-135. https://doi.org/10.1136/jech.2005.039792.
4. Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137(3):642-650. https://doi.org/10.1378/chest.09-0345.
5. Flores G, Tomany-Korman SC, Corey CR, Freeman HE, Shapiro MF. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121(2):e286-98. https://doi.org/10.1542/peds.2007-1243.
6. Fedele DA, Molzon ES, Eddington AR, Hullmann SE, Mullins LL, Gillaspy SG. Perceived barriers to care in a pediatric medical home: the moderating role of caregiver minority status. Clin Pediatr (Phila). 2014;53(4):351-355. https://doi.org/10.1177/0009922813507994.
7. Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children’s health. Pediatrics. 2008;121(2):337-344. https://doi.org/10.1542/peds.2007-0447.
8. Bauman LJ, Silver EJ, Stein REK. Cumulative social disadvantage and child health. Pediatrics. 2006;117(4):1321-1328. https://doi.org/10.1542/peds.2005-1647.
9. Andrulis DP. Moving beyond the status quo in reducing racial and ethnic disparities in children’s health. Public Health Rep. 2005;120(4):370-377. https://doi.org/10.1177/003335490512000403.
10. Flores G, Lin H. Trends in racial/ethnic disparities in medical and oral health, access to care, and use of services in US children: has anything changed over the years? Int J Equity Health. 2013;12:10. https://doi.org/10.1186/1475-9276-12-10.
11. Seid M, Stevens GD, Varni JW. Parents’ perceptions of pediatric primary care quality: effects of race/ethnicity, language, and access. Health Serv Res. 2003;38(4):1009-1031. https://doi.org/10.1111/1475-6773.00160.
12. Seid M, Varni JW, Cummings L, Schonlau M. The impact of realized access to care on health-related quality of life: a two-year prospective cohort study of children in the California State Children’s Health Insurance Program. J Pediatr. 2006;149(3):354-361. https://doi.org/10.1016/j.jpeds.2006.04.024.
13. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. https://doi.xorg/10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2.
14. Simon AE, Chan KS, Forrest CB. Assessment of children’s health-related quality of life in the united states with a multidimensional index. Pediatrics. 2008;121(1):e118-e126. https://doi.org/10.1542/peds.2007-0480.
15. Cheng TL, Emmanuel MA, Levy DJ, Jenkins RR. Child health disparities: what can a clinician do? Pediatrics. 2015;136(5):961-968. https://doi.org/10.1542/peds.2014-4126.
16. Christakis DA, Mell L, Koepsell TD, Zimmerman FJ, Connell FA. Association of lower continuity of care with greater risk of emergency department use and hospitalization in children. Pediatrics. 2001;107(3):524-529. https://doi.org/10.1542/peds.107.3.524.
17. Lion KC, Raphael JL. Partnering health disparities research with quality improvement science in pediatrics. Pediatrics. 2015;135(2):354-361. https://doi.org/10.1542/peds.2014-2982.
18. Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14:S8-S17. https://doi.org/10.1097/01.PHH.0000338382.36695.42.
19. Beck AF, Cohen AJ, Colvin JD, et al. Perspectives from the Society for Pediatric Research: interventions targeting social needs in pediatric clinical care. Pediatr Res. 2018;84(1):10-21. https://doi.org/10.1038/s41390-018-0012-1.
20. Shah AN, Simmons J, Beck AF. Adding a vital sign: considering the utility of place-based measures in health care settings. Hosp Pediatr. 2018;8(2):112-114. https://doi.org/10.1542/hpeds.2017-0219.
21. Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
22. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. https://doi.org/10.1001/jamapediatrics.2014.1600.
23. Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and health-related quality of life after pediatric inpatient surgery. J Pain. 2015;16(12):1334-1341. https://doi.org/10.1016/j.jpain.2015.09.005.
24. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric respiratory illness measurement system (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242.
25. Child and Adolescent Health Measurement Initiative. National survey of children with special health care needs (NS-CSHCN), 2009-2010. Available at: http://childhealthdata.org/learn/NS-CSHCN/topics_questions. Accessed on September 20, 2018.
26. Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:1. https://doi.org/10.1186/1477-7525-5-1.
27. Buuren S van, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. https://doi.org/10.18637/jss.v045.i03.
28. Elliott MN, Haviland AM, Kanouse DE, Hambarsoomian K, Hays RD. Adjusting for subgroup differences in extreme response tendency in ratings of health care: impact on disparity estimates. Heal Serv Res. 2009;44(2 Pt 1):542-561. https://doi.org/10.1111/j.1475-6773.2008.00922.x.
29. Stevens GD, Vane C, Cousineau MR. Association of experiences of medical home quality with health-related quality of life and school engagement among Latino children in low-income families. Health Serv Res. 2011;46(6pt1):1822-1842. https://doi.org/10.1111/j.1475-6773.2011.01292.x.
30. Long WE, Bauchner H, Sege RD, Cabral HJ, Garg A. The value of the medical home for children without special health care needs. Pediatrics. 2012;129(1):87-98. https://doi.org/10.1542/peds.2011-1739.
31. Strickland BB, Jones JR, Ghandour RM, Kogan MD, Newacheck PW. The medical home: health care access and impact for children and youth in the United States. Pediatrics. 2011;127(4):604-611. https://doi.org/10.1542/peds.2009-3555.
32. Sokol R, Austin A, Chandler C, et al. Screening children for social determinants of health: a systematic review. Pediatrics. 2019;144(4):e20191622. https://doi.org/10.1542/peds.2019-1622.
© 2020 Society of Hospital Medicine
Collective Action and Effective Dialogue to Address Gender Bias in Medicine
In 2016, Pediatric Hospital Medicine (PHM) was recognized as a subspecialty under the American Board of Pediatrics (ABP), one of 24 certifying boards of the American Board of Medical Specialties. As with all new ABP subspecialty certification processes, a “practice pathway” with specific eligibility criteria allows individuals with expertise and sufficient practice experience within the discipline to take the certification examination. For PHM, certification via the practice pathway is permissible for the 2019, 2021, and 2023 certifying examinations.1 In this perspective, we provide an illustration of ABP leadership and the PHM community partnering to mitigate unintentional gender bias that surfaced after the practice pathway eligibility criteria were implemented. We also provide recommendations to revise these criteria to eliminate future gender bias and promote equity in medicine.
In July 2019, individuals within the PHM community began to share stories of being denied eligibility to sit for the 2019 exam.2 Some of the reported denials were due to an eligibility criterion related to “practice interruptions”, which stated that practice interruptions cannot exceed three months in the preceding four years or six months in the preceding five years. Notably, some women reported that their applications were denied because of practice interruptions due to maternity leave. These stories raised significant concerns of gender bias in the board certification process and sparked collective action to revise the board certification eligibility criteria. A petition was circulated within the PHM community and received 1,479 signatures in two weeks.
Given the magnitude of concern, leaders within the PHM community, with support from the American Academy of Pediatrics, collaboratively engaged with the ABP and members of the ABP PHM subboard to improve the transparency and equity of the eligibility process. As a result of this activism and effective dialogue, the ABP revised the PHM board certification eligibility criteria and removed the practice interruption criterion.1 Through this unique experience of advocacy and partner
Gender bias is defined as the unfair difference in the way men and women are treated.3 Maternal bias is further characterized as bias experienced by mothers related to motherhood, often involving discrimination based on pregnancy, maternity leave, or breastfeeding. Both are common in medicine. Two-thirds of physician mothers report experiencing gender bias and more than a third experience maternal bias.4 This bias may be explicit, or intentional, but often the bias is unintentional. This bias can occur even with equal representation of women and men on committees determining eligibility, and even when the committee believes it is not biased.5 Furthermore, gender or maternal bias negatively affects individuals in medicine in regards to future employment, career advancement, and compensation.6-11
Given these implications, we celebrate the removal of the practice interruptions criterion as it was unintentionally biased against women. Eligibility criteria that considered practice interruptions would have disproportionately affected women due to leaves related to pregnancy and due to discrepancies in the length of parental leave for mothers versus fathers. Though the ABP’s initial review of cases of denial did not demonstrate a significant difference in the proportion of men and women who were denied, these data may be misleading. Potential reasons why the ABP did not find significant differences in denial rates between women and men include: (1) some women who had recent maternity leaves chose not to apply because of concerns they may be denied; or (2) some women did not disclose maternity leaves on their application because they did not interpret maternity leave to be a practice interruption. This “self-censoring” may have resulted in incomplete data, making it difficult to fully understand the differential impact of this criterion on women versus men. Therefore, it is essential that we as a profession continue to identify any areas where gender bias exists in determining eligibility for certification, employment, or career advancement within medicine and eliminate it.
Despite the improvements made in the revised criteria, further revision is necessary to remove the criterion related to the “start date”, which will differentially affect women. This criterion states that an individual must have started their PHM practice on or before July of the first year of a four-year look-back period (eg, July 2015 for the 2019 cycle). We present three theoretical cases to illustrate gender bias with respect to this criterion (Table). Even though Applicants #2 and #3 accrue far more than the minimum number of hours in their first year—and more hours overall than Applicant #1—both of these women will remain ineligible under the revised criteria. While Applicant #2 could be eligible for the 2021 or 2023 cycle, Applicant #3, who is new to PHM practice in 2019 as a residency graduate, will not be eligible at all under the practice pathway due to delayed graduation from residency.
Parental leave during residency following birth of a child may result in the need to make up the time missed.12 This means that more women than men will experience delayed entry into the workforce due to late graduation from residency.13 Women who experience a gap in employment at the start of their PHM practice due to pregnancy or childbirth will also be differentially affected by this criterion. If this same type of gap were to occur later in the year, it would no longer impact a woman’s eligibility under the revised criteria. Therefore, we implore the ABP to reevaluate this criterion which results in a hidden “practice interruption” penalty. Removing eligibility criteria related to practice interruptions, wherever they may occur, will not only eliminate systematic bias against women, but may also encourage men to take paternity leave, for which the benefits to both men and women are well described.14,15
We support the ABP’s mission to maintain the public’s trust by ensuring PHM board certification is an indicator that individuals have met a high standard. We acknowledge that the ABP and PHM subboard had to draw a line to create minimum standards. The start date and four-year look-back criteria were informed by prior certification processes, and the PHM community was given the opportunity to comment on these criteria prior to final ABP approval. However, now that we have become aware of how the start date criteria can differentially impact women and men, we must reevaluate this line to ensure that women and men are treated equally. Similar to the removal of the practice interruptions criterion, we do not believe that removal of the start date criterion will in any way compromise these standards. A four-year look-back period will still be in place and individuals will still be required to accrue the minimum number of hours in the first year and each subsequent year of the four-year period.
Despite any change in the criteria, there will be individuals who remain ineligible for PHM board certification. We will need to rely on institutions and the societies that lead PHM to remember that not all individuals had the opportunity to certify as a pediatric hospitalist, and for some, this was due to maternity leave. No woman should have to worry about her future employment when considering motherhood.
We hope the lessons learned from this experience will be informative for other specialties considering a new certification. Committees designing new criteria should have proportional representation of women and men, inclusion of underrepresented minorities, and members with a range of ages, orientations, identities, and abilities. Criteria should be closely scrutinized to evaluate if a single group of people is more likely to be excluded. All application reviewers should undergo training in identifying implicit bias.16 Once eligibility criteria are determined, they should be transparent to all applicants, consistently applied, and decisions to applicants should clearly state which criteria were or were not met. Regular audits should be conducted to identify any bias. Finally, transparent and respectful dialogue between the certifying board and the physician community is paramount to ensuring continuous quality improvement in the process.
The PHM experience with this new board certification process highlights the positive impact that the PHM community had engaging with the ABP leadership, who listened to the concerns and revised the eligibility criteria. We are optimistic that this productive relationship will continue to eliminate any gender bias in the board certification process. In turn, PHM and the ABP can be leaders in ending gender inequity in medicine.
Disclosures
The authors have nothing to disclose.
1. Nichols DG, Woods SK. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322
2. Don’t make me choose between motherhood and my career. https://www.kevinmd.com/blog/2019/08/dont-make-me-choose-between-motherhood-and-my-career.html. Accessed September 16, 2019.
3. GENDER BIAS | definition in the Cambridge English Dictionary. April 2019. https://dictionary.cambridge.org/us/dictionary/english/gender-bias.
4. Adesoye T, Mangurian C, Choo EK, Girgis C, Sabry-Elnaggar H, Linos E. Perceived discrimination experienced by physician mothers and desired workplace changes: A cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
5. Régner I, Thinus-Blanc C, Netter A, Schmader T, Huguet P. Committees with implicit biases promote fewer women when they do not believe gender bias exists. Nat Hum Behav. 2019. https://doi.org/10.1038/s41562-019-0686-3
6. Trix F, Psenka C. Exploring the color of glass: Letters of recommendation for female and male medical faculty. Discourse Soc. 2003;14(2):191-220. https://doi.org/10.1177/0957926503014002277
7. Correll SJ, Benard S, Paik I. Getting a job: Is there a motherhood penalty? Am J Sociol. 2007;112(5):1297-1339. https://doi.org/10.1086/511799
8. Aamc. Analysis in Brief - August 2009: Unconscious Bias in Faculty and Leadership Recruitment: A Literature Review; 2009. https://implicit.harvard.edu/. Accessed September 10, 2019.
9. Wright AL, Schwindt LA, Bassford TL, et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78(5):500-508. https://doi.org/10.1097/00001888-200305000-00015
10. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
11. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. September 2019:e20183955. https://doi.org/10.1542/peds.2018-3955
12. Section on Medical Students, Residents and Fellowship Trainees, Committee on Early Childhood. Parental leave for residents and pediatric training programs. Pediatrics. 2013;131(2):387-390. https://doi.org/10.1542/peds.2012-3542
13. Jagsi R, Tarbell NJ, Weinstein DF. Becoming a doctor, starting a family — leaves of absence from graduate medical education. N Engl J Med. 2007;357(19):1889-1891. https://doi.org/10.1056/NEJMp078163
14. Nepomnyaschy L, Waldfogel J. Paternity leave and fathers’ involvement with their young children. Community Work Fam. 2007;10(4):427-453. https://doi.org/10.1080/13668800701575077
15. Andersen SH. Paternity leave and the motherhood penalty: New causal evidence. J Marriage Fam. 2018;80(5):1125-1143. https://doi.org/10.1111/jomf.12507.
16. Girod S, Fassiotto M, Grewal D, et al. Reducing Implicit Gender Leadership Bias in Academic Medicine With an Educational Intervention. Acad Med. 2016;91(8):1143-1150. https://doi.org/10.1097/ACM.0000000000001099
In 2016, Pediatric Hospital Medicine (PHM) was recognized as a subspecialty under the American Board of Pediatrics (ABP), one of 24 certifying boards of the American Board of Medical Specialties. As with all new ABP subspecialty certification processes, a “practice pathway” with specific eligibility criteria allows individuals with expertise and sufficient practice experience within the discipline to take the certification examination. For PHM, certification via the practice pathway is permissible for the 2019, 2021, and 2023 certifying examinations.1 In this perspective, we provide an illustration of ABP leadership and the PHM community partnering to mitigate unintentional gender bias that surfaced after the practice pathway eligibility criteria were implemented. We also provide recommendations to revise these criteria to eliminate future gender bias and promote equity in medicine.
In July 2019, individuals within the PHM community began to share stories of being denied eligibility to sit for the 2019 exam.2 Some of the reported denials were due to an eligibility criterion related to “practice interruptions”, which stated that practice interruptions cannot exceed three months in the preceding four years or six months in the preceding five years. Notably, some women reported that their applications were denied because of practice interruptions due to maternity leave. These stories raised significant concerns of gender bias in the board certification process and sparked collective action to revise the board certification eligibility criteria. A petition was circulated within the PHM community and received 1,479 signatures in two weeks.
Given the magnitude of concern, leaders within the PHM community, with support from the American Academy of Pediatrics, collaboratively engaged with the ABP and members of the ABP PHM subboard to improve the transparency and equity of the eligibility process. As a result of this activism and effective dialogue, the ABP revised the PHM board certification eligibility criteria and removed the practice interruption criterion.1 Through this unique experience of advocacy and partner
Gender bias is defined as the unfair difference in the way men and women are treated.3 Maternal bias is further characterized as bias experienced by mothers related to motherhood, often involving discrimination based on pregnancy, maternity leave, or breastfeeding. Both are common in medicine. Two-thirds of physician mothers report experiencing gender bias and more than a third experience maternal bias.4 This bias may be explicit, or intentional, but often the bias is unintentional. This bias can occur even with equal representation of women and men on committees determining eligibility, and even when the committee believes it is not biased.5 Furthermore, gender or maternal bias negatively affects individuals in medicine in regards to future employment, career advancement, and compensation.6-11
Given these implications, we celebrate the removal of the practice interruptions criterion as it was unintentionally biased against women. Eligibility criteria that considered practice interruptions would have disproportionately affected women due to leaves related to pregnancy and due to discrepancies in the length of parental leave for mothers versus fathers. Though the ABP’s initial review of cases of denial did not demonstrate a significant difference in the proportion of men and women who were denied, these data may be misleading. Potential reasons why the ABP did not find significant differences in denial rates between women and men include: (1) some women who had recent maternity leaves chose not to apply because of concerns they may be denied; or (2) some women did not disclose maternity leaves on their application because they did not interpret maternity leave to be a practice interruption. This “self-censoring” may have resulted in incomplete data, making it difficult to fully understand the differential impact of this criterion on women versus men. Therefore, it is essential that we as a profession continue to identify any areas where gender bias exists in determining eligibility for certification, employment, or career advancement within medicine and eliminate it.
Despite the improvements made in the revised criteria, further revision is necessary to remove the criterion related to the “start date”, which will differentially affect women. This criterion states that an individual must have started their PHM practice on or before July of the first year of a four-year look-back period (eg, July 2015 for the 2019 cycle). We present three theoretical cases to illustrate gender bias with respect to this criterion (Table). Even though Applicants #2 and #3 accrue far more than the minimum number of hours in their first year—and more hours overall than Applicant #1—both of these women will remain ineligible under the revised criteria. While Applicant #2 could be eligible for the 2021 or 2023 cycle, Applicant #3, who is new to PHM practice in 2019 as a residency graduate, will not be eligible at all under the practice pathway due to delayed graduation from residency.
Parental leave during residency following birth of a child may result in the need to make up the time missed.12 This means that more women than men will experience delayed entry into the workforce due to late graduation from residency.13 Women who experience a gap in employment at the start of their PHM practice due to pregnancy or childbirth will also be differentially affected by this criterion. If this same type of gap were to occur later in the year, it would no longer impact a woman’s eligibility under the revised criteria. Therefore, we implore the ABP to reevaluate this criterion which results in a hidden “practice interruption” penalty. Removing eligibility criteria related to practice interruptions, wherever they may occur, will not only eliminate systematic bias against women, but may also encourage men to take paternity leave, for which the benefits to both men and women are well described.14,15
We support the ABP’s mission to maintain the public’s trust by ensuring PHM board certification is an indicator that individuals have met a high standard. We acknowledge that the ABP and PHM subboard had to draw a line to create minimum standards. The start date and four-year look-back criteria were informed by prior certification processes, and the PHM community was given the opportunity to comment on these criteria prior to final ABP approval. However, now that we have become aware of how the start date criteria can differentially impact women and men, we must reevaluate this line to ensure that women and men are treated equally. Similar to the removal of the practice interruptions criterion, we do not believe that removal of the start date criterion will in any way compromise these standards. A four-year look-back period will still be in place and individuals will still be required to accrue the minimum number of hours in the first year and each subsequent year of the four-year period.
Despite any change in the criteria, there will be individuals who remain ineligible for PHM board certification. We will need to rely on institutions and the societies that lead PHM to remember that not all individuals had the opportunity to certify as a pediatric hospitalist, and for some, this was due to maternity leave. No woman should have to worry about her future employment when considering motherhood.
We hope the lessons learned from this experience will be informative for other specialties considering a new certification. Committees designing new criteria should have proportional representation of women and men, inclusion of underrepresented minorities, and members with a range of ages, orientations, identities, and abilities. Criteria should be closely scrutinized to evaluate if a single group of people is more likely to be excluded. All application reviewers should undergo training in identifying implicit bias.16 Once eligibility criteria are determined, they should be transparent to all applicants, consistently applied, and decisions to applicants should clearly state which criteria were or were not met. Regular audits should be conducted to identify any bias. Finally, transparent and respectful dialogue between the certifying board and the physician community is paramount to ensuring continuous quality improvement in the process.
The PHM experience with this new board certification process highlights the positive impact that the PHM community had engaging with the ABP leadership, who listened to the concerns and revised the eligibility criteria. We are optimistic that this productive relationship will continue to eliminate any gender bias in the board certification process. In turn, PHM and the ABP can be leaders in ending gender inequity in medicine.
Disclosures
The authors have nothing to disclose.
In 2016, Pediatric Hospital Medicine (PHM) was recognized as a subspecialty under the American Board of Pediatrics (ABP), one of 24 certifying boards of the American Board of Medical Specialties. As with all new ABP subspecialty certification processes, a “practice pathway” with specific eligibility criteria allows individuals with expertise and sufficient practice experience within the discipline to take the certification examination. For PHM, certification via the practice pathway is permissible for the 2019, 2021, and 2023 certifying examinations.1 In this perspective, we provide an illustration of ABP leadership and the PHM community partnering to mitigate unintentional gender bias that surfaced after the practice pathway eligibility criteria were implemented. We also provide recommendations to revise these criteria to eliminate future gender bias and promote equity in medicine.
In July 2019, individuals within the PHM community began to share stories of being denied eligibility to sit for the 2019 exam.2 Some of the reported denials were due to an eligibility criterion related to “practice interruptions”, which stated that practice interruptions cannot exceed three months in the preceding four years or six months in the preceding five years. Notably, some women reported that their applications were denied because of practice interruptions due to maternity leave. These stories raised significant concerns of gender bias in the board certification process and sparked collective action to revise the board certification eligibility criteria. A petition was circulated within the PHM community and received 1,479 signatures in two weeks.
Given the magnitude of concern, leaders within the PHM community, with support from the American Academy of Pediatrics, collaboratively engaged with the ABP and members of the ABP PHM subboard to improve the transparency and equity of the eligibility process. As a result of this activism and effective dialogue, the ABP revised the PHM board certification eligibility criteria and removed the practice interruption criterion.1 Through this unique experience of advocacy and partner
Gender bias is defined as the unfair difference in the way men and women are treated.3 Maternal bias is further characterized as bias experienced by mothers related to motherhood, often involving discrimination based on pregnancy, maternity leave, or breastfeeding. Both are common in medicine. Two-thirds of physician mothers report experiencing gender bias and more than a third experience maternal bias.4 This bias may be explicit, or intentional, but often the bias is unintentional. This bias can occur even with equal representation of women and men on committees determining eligibility, and even when the committee believes it is not biased.5 Furthermore, gender or maternal bias negatively affects individuals in medicine in regards to future employment, career advancement, and compensation.6-11
Given these implications, we celebrate the removal of the practice interruptions criterion as it was unintentionally biased against women. Eligibility criteria that considered practice interruptions would have disproportionately affected women due to leaves related to pregnancy and due to discrepancies in the length of parental leave for mothers versus fathers. Though the ABP’s initial review of cases of denial did not demonstrate a significant difference in the proportion of men and women who were denied, these data may be misleading. Potential reasons why the ABP did not find significant differences in denial rates between women and men include: (1) some women who had recent maternity leaves chose not to apply because of concerns they may be denied; or (2) some women did not disclose maternity leaves on their application because they did not interpret maternity leave to be a practice interruption. This “self-censoring” may have resulted in incomplete data, making it difficult to fully understand the differential impact of this criterion on women versus men. Therefore, it is essential that we as a profession continue to identify any areas where gender bias exists in determining eligibility for certification, employment, or career advancement within medicine and eliminate it.
Despite the improvements made in the revised criteria, further revision is necessary to remove the criterion related to the “start date”, which will differentially affect women. This criterion states that an individual must have started their PHM practice on or before July of the first year of a four-year look-back period (eg, July 2015 for the 2019 cycle). We present three theoretical cases to illustrate gender bias with respect to this criterion (Table). Even though Applicants #2 and #3 accrue far more than the minimum number of hours in their first year—and more hours overall than Applicant #1—both of these women will remain ineligible under the revised criteria. While Applicant #2 could be eligible for the 2021 or 2023 cycle, Applicant #3, who is new to PHM practice in 2019 as a residency graduate, will not be eligible at all under the practice pathway due to delayed graduation from residency.
Parental leave during residency following birth of a child may result in the need to make up the time missed.12 This means that more women than men will experience delayed entry into the workforce due to late graduation from residency.13 Women who experience a gap in employment at the start of their PHM practice due to pregnancy or childbirth will also be differentially affected by this criterion. If this same type of gap were to occur later in the year, it would no longer impact a woman’s eligibility under the revised criteria. Therefore, we implore the ABP to reevaluate this criterion which results in a hidden “practice interruption” penalty. Removing eligibility criteria related to practice interruptions, wherever they may occur, will not only eliminate systematic bias against women, but may also encourage men to take paternity leave, for which the benefits to both men and women are well described.14,15
We support the ABP’s mission to maintain the public’s trust by ensuring PHM board certification is an indicator that individuals have met a high standard. We acknowledge that the ABP and PHM subboard had to draw a line to create minimum standards. The start date and four-year look-back criteria were informed by prior certification processes, and the PHM community was given the opportunity to comment on these criteria prior to final ABP approval. However, now that we have become aware of how the start date criteria can differentially impact women and men, we must reevaluate this line to ensure that women and men are treated equally. Similar to the removal of the practice interruptions criterion, we do not believe that removal of the start date criterion will in any way compromise these standards. A four-year look-back period will still be in place and individuals will still be required to accrue the minimum number of hours in the first year and each subsequent year of the four-year period.
Despite any change in the criteria, there will be individuals who remain ineligible for PHM board certification. We will need to rely on institutions and the societies that lead PHM to remember that not all individuals had the opportunity to certify as a pediatric hospitalist, and for some, this was due to maternity leave. No woman should have to worry about her future employment when considering motherhood.
We hope the lessons learned from this experience will be informative for other specialties considering a new certification. Committees designing new criteria should have proportional representation of women and men, inclusion of underrepresented minorities, and members with a range of ages, orientations, identities, and abilities. Criteria should be closely scrutinized to evaluate if a single group of people is more likely to be excluded. All application reviewers should undergo training in identifying implicit bias.16 Once eligibility criteria are determined, they should be transparent to all applicants, consistently applied, and decisions to applicants should clearly state which criteria were or were not met. Regular audits should be conducted to identify any bias. Finally, transparent and respectful dialogue between the certifying board and the physician community is paramount to ensuring continuous quality improvement in the process.
The PHM experience with this new board certification process highlights the positive impact that the PHM community had engaging with the ABP leadership, who listened to the concerns and revised the eligibility criteria. We are optimistic that this productive relationship will continue to eliminate any gender bias in the board certification process. In turn, PHM and the ABP can be leaders in ending gender inequity in medicine.
Disclosures
The authors have nothing to disclose.
1. Nichols DG, Woods SK. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322
2. Don’t make me choose between motherhood and my career. https://www.kevinmd.com/blog/2019/08/dont-make-me-choose-between-motherhood-and-my-career.html. Accessed September 16, 2019.
3. GENDER BIAS | definition in the Cambridge English Dictionary. April 2019. https://dictionary.cambridge.org/us/dictionary/english/gender-bias.
4. Adesoye T, Mangurian C, Choo EK, Girgis C, Sabry-Elnaggar H, Linos E. Perceived discrimination experienced by physician mothers and desired workplace changes: A cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
5. Régner I, Thinus-Blanc C, Netter A, Schmader T, Huguet P. Committees with implicit biases promote fewer women when they do not believe gender bias exists. Nat Hum Behav. 2019. https://doi.org/10.1038/s41562-019-0686-3
6. Trix F, Psenka C. Exploring the color of glass: Letters of recommendation for female and male medical faculty. Discourse Soc. 2003;14(2):191-220. https://doi.org/10.1177/0957926503014002277
7. Correll SJ, Benard S, Paik I. Getting a job: Is there a motherhood penalty? Am J Sociol. 2007;112(5):1297-1339. https://doi.org/10.1086/511799
8. Aamc. Analysis in Brief - August 2009: Unconscious Bias in Faculty and Leadership Recruitment: A Literature Review; 2009. https://implicit.harvard.edu/. Accessed September 10, 2019.
9. Wright AL, Schwindt LA, Bassford TL, et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78(5):500-508. https://doi.org/10.1097/00001888-200305000-00015
10. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
11. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. September 2019:e20183955. https://doi.org/10.1542/peds.2018-3955
12. Section on Medical Students, Residents and Fellowship Trainees, Committee on Early Childhood. Parental leave for residents and pediatric training programs. Pediatrics. 2013;131(2):387-390. https://doi.org/10.1542/peds.2012-3542
13. Jagsi R, Tarbell NJ, Weinstein DF. Becoming a doctor, starting a family — leaves of absence from graduate medical education. N Engl J Med. 2007;357(19):1889-1891. https://doi.org/10.1056/NEJMp078163
14. Nepomnyaschy L, Waldfogel J. Paternity leave and fathers’ involvement with their young children. Community Work Fam. 2007;10(4):427-453. https://doi.org/10.1080/13668800701575077
15. Andersen SH. Paternity leave and the motherhood penalty: New causal evidence. J Marriage Fam. 2018;80(5):1125-1143. https://doi.org/10.1111/jomf.12507.
16. Girod S, Fassiotto M, Grewal D, et al. Reducing Implicit Gender Leadership Bias in Academic Medicine With an Educational Intervention. Acad Med. 2016;91(8):1143-1150. https://doi.org/10.1097/ACM.0000000000001099
1. Nichols DG, Woods SK. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322
2. Don’t make me choose between motherhood and my career. https://www.kevinmd.com/blog/2019/08/dont-make-me-choose-between-motherhood-and-my-career.html. Accessed September 16, 2019.
3. GENDER BIAS | definition in the Cambridge English Dictionary. April 2019. https://dictionary.cambridge.org/us/dictionary/english/gender-bias.
4. Adesoye T, Mangurian C, Choo EK, Girgis C, Sabry-Elnaggar H, Linos E. Perceived discrimination experienced by physician mothers and desired workplace changes: A cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
5. Régner I, Thinus-Blanc C, Netter A, Schmader T, Huguet P. Committees with implicit biases promote fewer women when they do not believe gender bias exists. Nat Hum Behav. 2019. https://doi.org/10.1038/s41562-019-0686-3
6. Trix F, Psenka C. Exploring the color of glass: Letters of recommendation for female and male medical faculty. Discourse Soc. 2003;14(2):191-220. https://doi.org/10.1177/0957926503014002277
7. Correll SJ, Benard S, Paik I. Getting a job: Is there a motherhood penalty? Am J Sociol. 2007;112(5):1297-1339. https://doi.org/10.1086/511799
8. Aamc. Analysis in Brief - August 2009: Unconscious Bias in Faculty and Leadership Recruitment: A Literature Review; 2009. https://implicit.harvard.edu/. Accessed September 10, 2019.
9. Wright AL, Schwindt LA, Bassford TL, et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78(5):500-508. https://doi.org/10.1097/00001888-200305000-00015
10. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
11. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. September 2019:e20183955. https://doi.org/10.1542/peds.2018-3955
12. Section on Medical Students, Residents and Fellowship Trainees, Committee on Early Childhood. Parental leave for residents and pediatric training programs. Pediatrics. 2013;131(2):387-390. https://doi.org/10.1542/peds.2012-3542
13. Jagsi R, Tarbell NJ, Weinstein DF. Becoming a doctor, starting a family — leaves of absence from graduate medical education. N Engl J Med. 2007;357(19):1889-1891. https://doi.org/10.1056/NEJMp078163
14. Nepomnyaschy L, Waldfogel J. Paternity leave and fathers’ involvement with their young children. Community Work Fam. 2007;10(4):427-453. https://doi.org/10.1080/13668800701575077
15. Andersen SH. Paternity leave and the motherhood penalty: New causal evidence. J Marriage Fam. 2018;80(5):1125-1143. https://doi.org/10.1111/jomf.12507.
16. Girod S, Fassiotto M, Grewal D, et al. Reducing Implicit Gender Leadership Bias in Academic Medicine With an Educational Intervention. Acad Med. 2016;91(8):1143-1150. https://doi.org/10.1097/ACM.0000000000001099
© 2019 Society of Hospital Medicine