User login
Epidemiology of gout
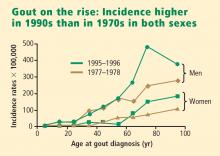
Both the incidence and the prevalence of gout appear to be increasing worldwide.1,2 Risk factors for the development of gout are related to our increasing longevity, dietary and lifestyle changes, and an increased prevalence of comorbid conditions. Patients with conditions such as hypertension, diabetes, cardiovascular disease, and the metabolic syndrome have an increased risk of developing hyperuricemia and gout,1,2 and such conditions are frequently managed by primary care physicians. This paper discusses how these conditions, along with diet, alcohol intake, and lifestyle, contribute to the increasing prevalence of hyperuricemia and gout.1
PREVALENCE AND INCIDENCE: BOTH ARE RISING
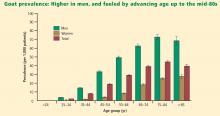
The Third National Health and Nutrition Examination Survey (NHANES III) estimated the prevalence of gout in the US population to be 5.1 million between 1988 and 1994.3 Data from a US managed care claims database revealed an increase in gout prevalence from 2.9 cases per 1,000 persons in 1990 to 5.2 cases per 1,000 persons in 1999.4 Other studies indicate that gout is becoming more prevalent in societies such as New Zealand and Taiwan as well as in the United States.5,6
The NHANES III data show that gout affected more than 3 million men aged 40 years or older, and 1.7 million women aged 40 years or older, in the period from 1988 to 1994.3 The estimated prevalence of gout among men aged 40 years or older makes this disease more common than rheumatoid arthritis and the most common form of inflammatory arthritis in adult men.7–9
RISK FACTORS FOR GOUT DEVELOPMENT
Sex and age
Men have higher serum urate levels than women do, which increases their risk of developing gout. Development of gout before age 30 years is overwhelmingly more likely in men than in women.7,9,10 The prevalence of gout in men increases with advancing age and peaks between ages 75 and 84 years.6 Women have an increased risk of developing gout after menopause; the risk begins to rise at about age 45 years with the decrease in estrogen levels.10,11 The incidence of gout becomes approximately equal between the sexes after age 60 years.10,12
It is important for clinicians to bear these factors in mind when taking the patient history and considering gout in the differential diagnosis. Although gout is more common in men, the diagnosis of gout should still be considered in women, particularly postmenopausal women.10,11
Medications
The use of diuretics is a significant risk factor for the development of gout. Diuretic use results in increased reabsorption of uric acid in the kidney, leading to hyperuricemia.1,7,10,13 If reasonable, an alternative antihypertensive agent should be prescribed. Low-dose aspirin, commonly prescribed for cardioprotection, also increases urate levels slightly in elderly patients,14 but should not be discontinued if indicated. Cyclosporine, which increases tubular reabsorption of urate,1 can result in a type of rapidly ascending gout that frequently is polyarticular.1,15 This is often encountered in transplant patients taking cyclosporine as an immunosuppressant. Hyperuricemia is also seen in patients taking pyrazinamide, ethambutol, and niacin. Physicians should be aware of the risks and benefits of prescribing any of these drugs to a patient with gout and consider that they may precipitate a gout flare in a previously unaffected patient.
Comorbidities
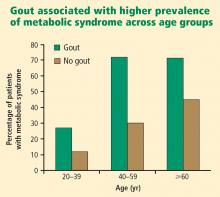
Primary care physicians regularly see patients with hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease, and the metabolic syndrome. As patients with these comorbidities are at an increased risk for developing gout,1,10,11 it may be beneficial in these patients to inquire whether they have had any bouts of arthritic pain and periodically evaluate the serum urate level (which is no longer included on chemistry profiles).
Obesity/high body mass index
Obesity and high body mass index significantly contribute to the risk of developing gout.13,16 Choi and colleagues observed that the risk of gout is very low for men with a body mass index between 21 and 22 but is increased threefold for men whose body mass index is 35 or greater.13 Obesity is associated with increased serum urate levels attributable to increased urate production and decreased renal urate excretion. A weight loss program may reduce the risk of gout by decreasing urate levels over time.13,16
Diet and alcohol consumption
A study by Choi and colleagues found that high intake of alcohol (beer more so than hard liquor or wine) and a diet rich in meat (particularly red meat, wild game, or organ meat) and seafood (particularly shellfish and some larger saltwater fish) increase the risk for developing gout.17,18 Purine-rich vegetables, which were previously eliminated in low-purine diets, were found not to have any association with hyperuricemia and did not increase the risk of gout.17,18 Frequent consumption of dairy products was found to slightly reduce the risk of gout and hyperuricemia.17,18
Although manipulation of diet and alcohol consumption alone rarely achieves the desired degree of serum urate reduction, adherence to changes in diet and alcohol intake will reduce flares of gout and assist in lowering serum urate levels.
CONCLUSIONS
Diet, alcohol consumption, and lifestyle choices can increase the risk of developing gout, but making recommended lifestyle changes does not replace pharmacologic treatments for existing gout or associated comorbidities. Furthermore, very few patients are likely to follow through with lifestyle changes such as weight reduction and a low-cholesterol diet.19–21 Therefore, physician awareness of the factors that contribute to the development of gout is important, as is identification of at-risk patients before they develop manifestations of the disease. Increased vigilance in monitoring serum urate levels and monitoring for gout development in at-risk patients may help to improve the standard of care for gout.
Acknowledgment
Dr. Weaver thanks Kenneth G. Saag, MD, MSc, for his contributions to the lecture on which this paper is based.
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol 2002; 29:2403–2406.
- Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Kidney Dis 2002; 40:37–42.
- Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004; 31: 1582–1587.
- Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol 2007; 3:443–449.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA 1991; 266:3004–3007.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004; 363:1277–1281.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998; 41:778–799.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2007; 57:109–115.
- Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol 1988; 41:237–242.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum 2000; 43:103–108.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001: 313–319.
- Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000; 59:539–543.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. Diet, alcohol, and gout: how do we advise patients given recent developments? Curr Rheumatol Rep 2005; 7:220–226.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Emmerson BT. The management of gout. N Engl J Med 1996; 334:445–451.
- Levinson W, Cohen MS, Brady D, Duffy FD. To change or not to change: “sounds like you have a dilemma.” Ann Intern Med 2001; 135:386–391.
Both the incidence and the prevalence of gout appear to be increasing worldwide.1,2 Risk factors for the development of gout are related to our increasing longevity, dietary and lifestyle changes, and an increased prevalence of comorbid conditions. Patients with conditions such as hypertension, diabetes, cardiovascular disease, and the metabolic syndrome have an increased risk of developing hyperuricemia and gout,1,2 and such conditions are frequently managed by primary care physicians. This paper discusses how these conditions, along with diet, alcohol intake, and lifestyle, contribute to the increasing prevalence of hyperuricemia and gout.1
PREVALENCE AND INCIDENCE: BOTH ARE RISING
The Third National Health and Nutrition Examination Survey (NHANES III) estimated the prevalence of gout in the US population to be 5.1 million between 1988 and 1994.3 Data from a US managed care claims database revealed an increase in gout prevalence from 2.9 cases per 1,000 persons in 1990 to 5.2 cases per 1,000 persons in 1999.4 Other studies indicate that gout is becoming more prevalent in societies such as New Zealand and Taiwan as well as in the United States.5,6
The NHANES III data show that gout affected more than 3 million men aged 40 years or older, and 1.7 million women aged 40 years or older, in the period from 1988 to 1994.3 The estimated prevalence of gout among men aged 40 years or older makes this disease more common than rheumatoid arthritis and the most common form of inflammatory arthritis in adult men.7–9
RISK FACTORS FOR GOUT DEVELOPMENT
Sex and age
Men have higher serum urate levels than women do, which increases their risk of developing gout. Development of gout before age 30 years is overwhelmingly more likely in men than in women.7,9,10 The prevalence of gout in men increases with advancing age and peaks between ages 75 and 84 years.6 Women have an increased risk of developing gout after menopause; the risk begins to rise at about age 45 years with the decrease in estrogen levels.10,11 The incidence of gout becomes approximately equal between the sexes after age 60 years.10,12
It is important for clinicians to bear these factors in mind when taking the patient history and considering gout in the differential diagnosis. Although gout is more common in men, the diagnosis of gout should still be considered in women, particularly postmenopausal women.10,11
Medications
The use of diuretics is a significant risk factor for the development of gout. Diuretic use results in increased reabsorption of uric acid in the kidney, leading to hyperuricemia.1,7,10,13 If reasonable, an alternative antihypertensive agent should be prescribed. Low-dose aspirin, commonly prescribed for cardioprotection, also increases urate levels slightly in elderly patients,14 but should not be discontinued if indicated. Cyclosporine, which increases tubular reabsorption of urate,1 can result in a type of rapidly ascending gout that frequently is polyarticular.1,15 This is often encountered in transplant patients taking cyclosporine as an immunosuppressant. Hyperuricemia is also seen in patients taking pyrazinamide, ethambutol, and niacin. Physicians should be aware of the risks and benefits of prescribing any of these drugs to a patient with gout and consider that they may precipitate a gout flare in a previously unaffected patient.
Comorbidities
Primary care physicians regularly see patients with hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease, and the metabolic syndrome. As patients with these comorbidities are at an increased risk for developing gout,1,10,11 it may be beneficial in these patients to inquire whether they have had any bouts of arthritic pain and periodically evaluate the serum urate level (which is no longer included on chemistry profiles).
Obesity/high body mass index
Obesity and high body mass index significantly contribute to the risk of developing gout.13,16 Choi and colleagues observed that the risk of gout is very low for men with a body mass index between 21 and 22 but is increased threefold for men whose body mass index is 35 or greater.13 Obesity is associated with increased serum urate levels attributable to increased urate production and decreased renal urate excretion. A weight loss program may reduce the risk of gout by decreasing urate levels over time.13,16
Diet and alcohol consumption
A study by Choi and colleagues found that high intake of alcohol (beer more so than hard liquor or wine) and a diet rich in meat (particularly red meat, wild game, or organ meat) and seafood (particularly shellfish and some larger saltwater fish) increase the risk for developing gout.17,18 Purine-rich vegetables, which were previously eliminated in low-purine diets, were found not to have any association with hyperuricemia and did not increase the risk of gout.17,18 Frequent consumption of dairy products was found to slightly reduce the risk of gout and hyperuricemia.17,18
Although manipulation of diet and alcohol consumption alone rarely achieves the desired degree of serum urate reduction, adherence to changes in diet and alcohol intake will reduce flares of gout and assist in lowering serum urate levels.
CONCLUSIONS
Diet, alcohol consumption, and lifestyle choices can increase the risk of developing gout, but making recommended lifestyle changes does not replace pharmacologic treatments for existing gout or associated comorbidities. Furthermore, very few patients are likely to follow through with lifestyle changes such as weight reduction and a low-cholesterol diet.19–21 Therefore, physician awareness of the factors that contribute to the development of gout is important, as is identification of at-risk patients before they develop manifestations of the disease. Increased vigilance in monitoring serum urate levels and monitoring for gout development in at-risk patients may help to improve the standard of care for gout.
Acknowledgment
Dr. Weaver thanks Kenneth G. Saag, MD, MSc, for his contributions to the lecture on which this paper is based.
Both the incidence and the prevalence of gout appear to be increasing worldwide.1,2 Risk factors for the development of gout are related to our increasing longevity, dietary and lifestyle changes, and an increased prevalence of comorbid conditions. Patients with conditions such as hypertension, diabetes, cardiovascular disease, and the metabolic syndrome have an increased risk of developing hyperuricemia and gout,1,2 and such conditions are frequently managed by primary care physicians. This paper discusses how these conditions, along with diet, alcohol intake, and lifestyle, contribute to the increasing prevalence of hyperuricemia and gout.1
PREVALENCE AND INCIDENCE: BOTH ARE RISING
The Third National Health and Nutrition Examination Survey (NHANES III) estimated the prevalence of gout in the US population to be 5.1 million between 1988 and 1994.3 Data from a US managed care claims database revealed an increase in gout prevalence from 2.9 cases per 1,000 persons in 1990 to 5.2 cases per 1,000 persons in 1999.4 Other studies indicate that gout is becoming more prevalent in societies such as New Zealand and Taiwan as well as in the United States.5,6
The NHANES III data show that gout affected more than 3 million men aged 40 years or older, and 1.7 million women aged 40 years or older, in the period from 1988 to 1994.3 The estimated prevalence of gout among men aged 40 years or older makes this disease more common than rheumatoid arthritis and the most common form of inflammatory arthritis in adult men.7–9
RISK FACTORS FOR GOUT DEVELOPMENT
Sex and age
Men have higher serum urate levels than women do, which increases their risk of developing gout. Development of gout before age 30 years is overwhelmingly more likely in men than in women.7,9,10 The prevalence of gout in men increases with advancing age and peaks between ages 75 and 84 years.6 Women have an increased risk of developing gout after menopause; the risk begins to rise at about age 45 years with the decrease in estrogen levels.10,11 The incidence of gout becomes approximately equal between the sexes after age 60 years.10,12
It is important for clinicians to bear these factors in mind when taking the patient history and considering gout in the differential diagnosis. Although gout is more common in men, the diagnosis of gout should still be considered in women, particularly postmenopausal women.10,11
Medications
The use of diuretics is a significant risk factor for the development of gout. Diuretic use results in increased reabsorption of uric acid in the kidney, leading to hyperuricemia.1,7,10,13 If reasonable, an alternative antihypertensive agent should be prescribed. Low-dose aspirin, commonly prescribed for cardioprotection, also increases urate levels slightly in elderly patients,14 but should not be discontinued if indicated. Cyclosporine, which increases tubular reabsorption of urate,1 can result in a type of rapidly ascending gout that frequently is polyarticular.1,15 This is often encountered in transplant patients taking cyclosporine as an immunosuppressant. Hyperuricemia is also seen in patients taking pyrazinamide, ethambutol, and niacin. Physicians should be aware of the risks and benefits of prescribing any of these drugs to a patient with gout and consider that they may precipitate a gout flare in a previously unaffected patient.
Comorbidities
Primary care physicians regularly see patients with hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease, and the metabolic syndrome. As patients with these comorbidities are at an increased risk for developing gout,1,10,11 it may be beneficial in these patients to inquire whether they have had any bouts of arthritic pain and periodically evaluate the serum urate level (which is no longer included on chemistry profiles).
Obesity/high body mass index
Obesity and high body mass index significantly contribute to the risk of developing gout.13,16 Choi and colleagues observed that the risk of gout is very low for men with a body mass index between 21 and 22 but is increased threefold for men whose body mass index is 35 or greater.13 Obesity is associated with increased serum urate levels attributable to increased urate production and decreased renal urate excretion. A weight loss program may reduce the risk of gout by decreasing urate levels over time.13,16
Diet and alcohol consumption
A study by Choi and colleagues found that high intake of alcohol (beer more so than hard liquor or wine) and a diet rich in meat (particularly red meat, wild game, or organ meat) and seafood (particularly shellfish and some larger saltwater fish) increase the risk for developing gout.17,18 Purine-rich vegetables, which were previously eliminated in low-purine diets, were found not to have any association with hyperuricemia and did not increase the risk of gout.17,18 Frequent consumption of dairy products was found to slightly reduce the risk of gout and hyperuricemia.17,18
Although manipulation of diet and alcohol consumption alone rarely achieves the desired degree of serum urate reduction, adherence to changes in diet and alcohol intake will reduce flares of gout and assist in lowering serum urate levels.
CONCLUSIONS
Diet, alcohol consumption, and lifestyle choices can increase the risk of developing gout, but making recommended lifestyle changes does not replace pharmacologic treatments for existing gout or associated comorbidities. Furthermore, very few patients are likely to follow through with lifestyle changes such as weight reduction and a low-cholesterol diet.19–21 Therefore, physician awareness of the factors that contribute to the development of gout is important, as is identification of at-risk patients before they develop manifestations of the disease. Increased vigilance in monitoring serum urate levels and monitoring for gout development in at-risk patients may help to improve the standard of care for gout.
Acknowledgment
Dr. Weaver thanks Kenneth G. Saag, MD, MSc, for his contributions to the lecture on which this paper is based.
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol 2002; 29:2403–2406.
- Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Kidney Dis 2002; 40:37–42.
- Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004; 31: 1582–1587.
- Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol 2007; 3:443–449.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA 1991; 266:3004–3007.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004; 363:1277–1281.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998; 41:778–799.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2007; 57:109–115.
- Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol 1988; 41:237–242.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum 2000; 43:103–108.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001: 313–319.
- Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000; 59:539–543.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. Diet, alcohol, and gout: how do we advise patients given recent developments? Curr Rheumatol Rep 2005; 7:220–226.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Emmerson BT. The management of gout. N Engl J Med 1996; 334:445–451.
- Levinson W, Cohen MS, Brady D, Duffy FD. To change or not to change: “sounds like you have a dilemma.” Ann Intern Med 2001; 135:386–391.
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005; 143:499–516.
- Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol 2002; 29:2403–2406.
- Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Kidney Dis 2002; 40:37–42.
- Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004; 31: 1582–1587.
- Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol 2007; 3:443–449.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA 1991; 266:3004–3007.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004; 363:1277–1281.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998; 41:778–799.
- Rott KT, Agudelo CA. Gout. JAMA 2003; 289:2857–2860.
- Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2007; 57:109–115.
- Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol 1988; 41:237–242.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum 2000; 43:103–108.
- Edwards NL. Gout. B. Clinical and laboratory features. In: Klippel JH, Crofford LJ, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001: 313–319.
- Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000; 59:539–543.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. Diet, alcohol, and gout: how do we advise patients given recent developments? Curr Rheumatol Rep 2005; 7:220–226.
- Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol 2005; 32:773–777.
- Emmerson BT. The management of gout. N Engl J Med 1996; 334:445–451.
- Levinson W, Cohen MS, Brady D, Duffy FD. To change or not to change: “sounds like you have a dilemma.” Ann Intern Med 2001; 135:386–391.
KEY POINTS
- Although gout is more common in men, the diagnosis of gout should still be considered in women, particularly postmenopausal women.
- Use of diuretics is a significant risk factor for gout.
- Patients with hypertension, diabetes, hyperlipidemia, chronic kidney disease, or the metabolic syndrome are at increased risk for developing gout. Vigilance for gout is especially indicated in patients with metabolic syndrome.