User login
Bleeding Hand Mass in an Older Man
The Diagnosis: Epithelioid Angiosarcoma
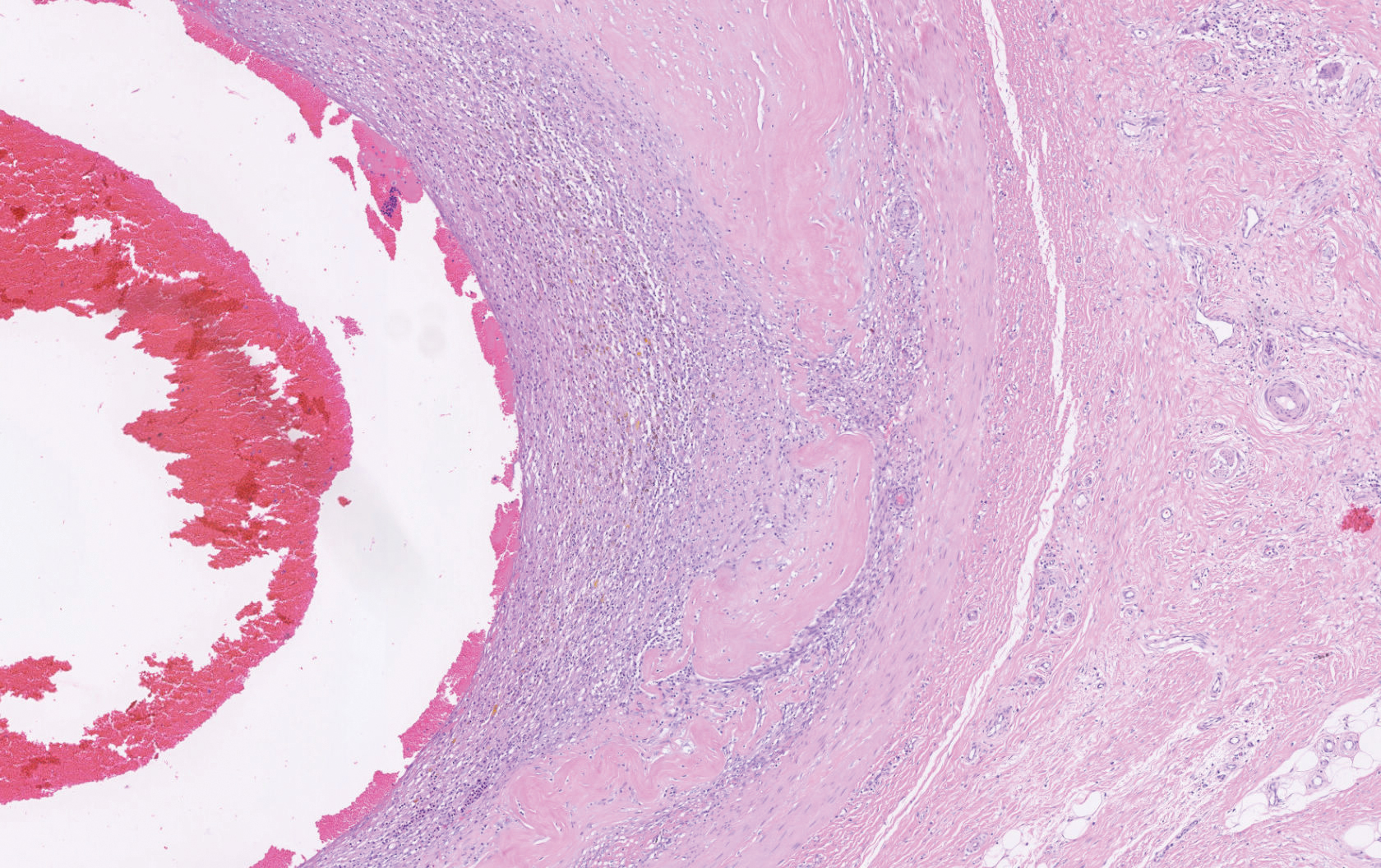
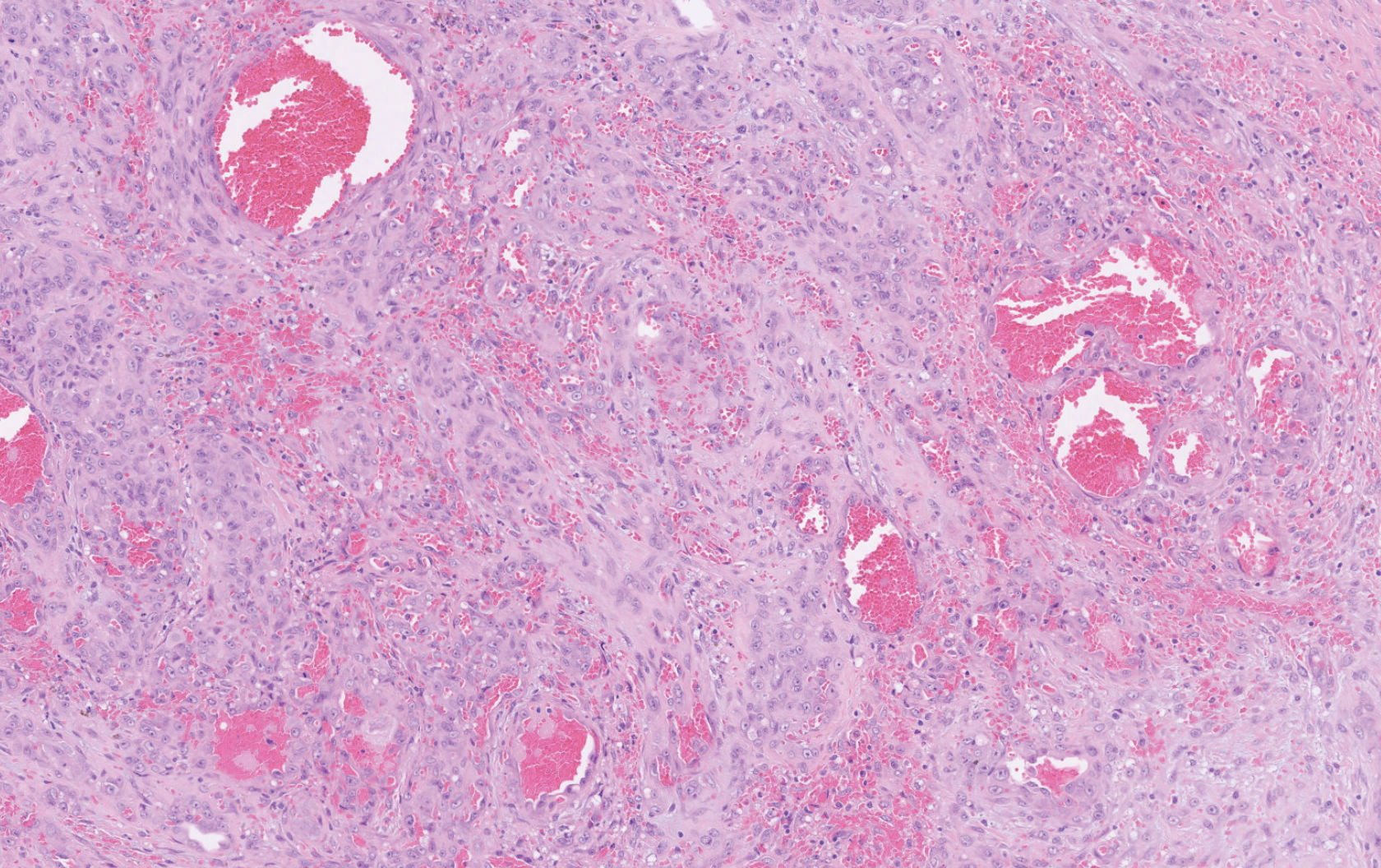
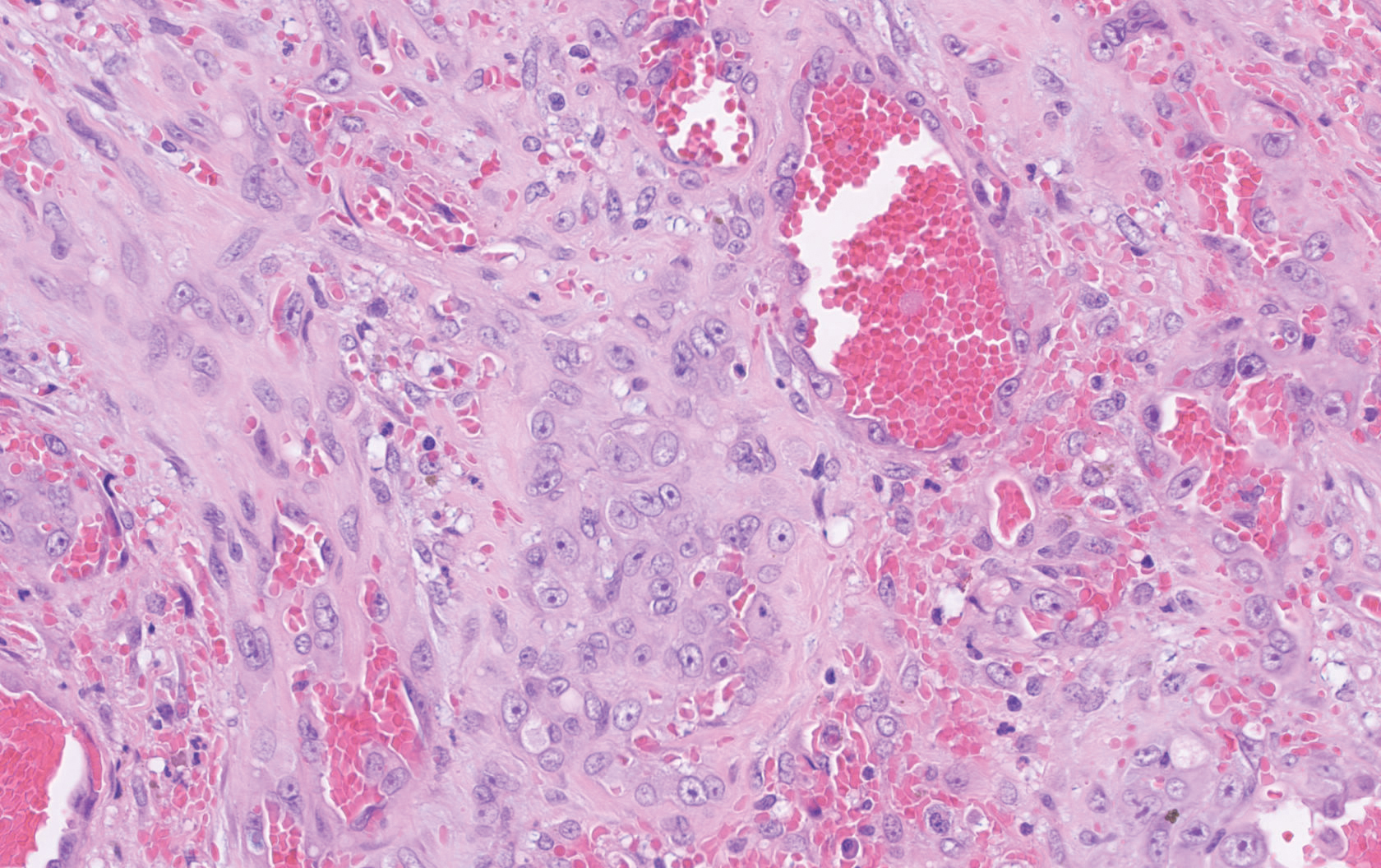
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
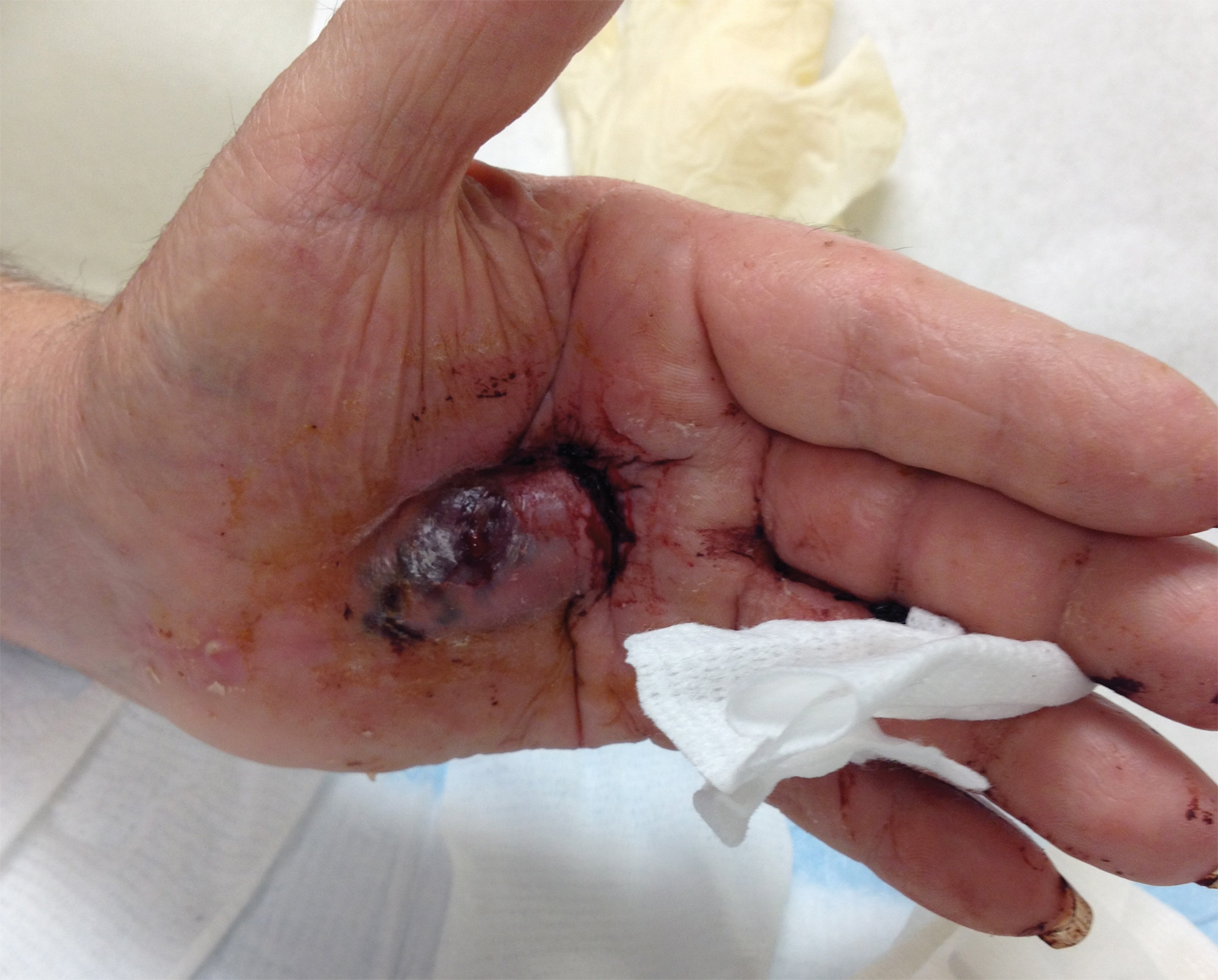
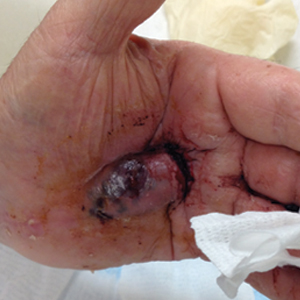
A 72-year-old man presented for evaluation of a mass on the left hand that continued to grow over the last few months and eventually bled. The patient first noticed a small firm lump on the palm approximately 1 year prior to presentation, and it was originally diagnosed as a Dupuytren contracture by his primary care physician. Months later, the lesion grew and began to bleed. Magnetic resonance imaging showed large hematomas of the hand with areas of nodular enhancement. The mass was located between the third and fourth proximal phalanges and abutted the extensor tendon. Complete excision yielded a definitive diagnosis.
Coalescing Hyperkeratotic Plaques and Papules
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5
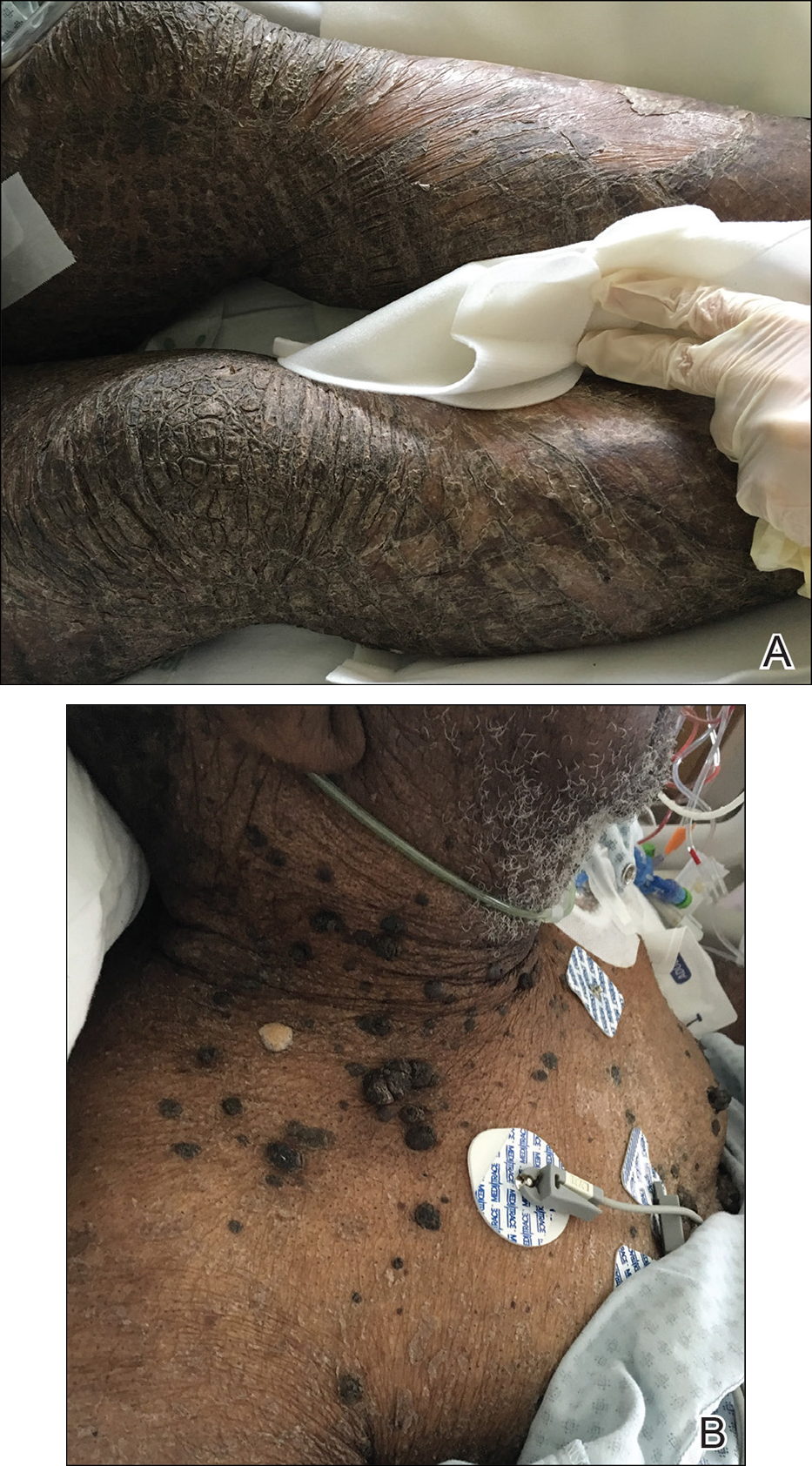
anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5

anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
The Diagnosis: X-Linked Ichthyosis
Immunohistochemical staining of a punch biopsy specimen from the left foot with cytokeratin markers AE1/3, 5/6, and 19 showed normal positive uptake. Further workup was recommended, and the patient was referred to genetics for an ichthyosis gene panel. DNA sequencing revealed a c.1121G>A transition in exon 10 of the steroid sulfatase gene, STS, consistent with X-linked ichthyosis (XLI).
X-linked ichthyosis, also known as steroid sulfatase deficiency and X-linked recessive ichthyosis, is a congenital skin disorder classified in 1965 by Wells and Kerr.1 Ichthyoses are a heterogenous group of acquired and congenital disorders of keratinization that manifest with xerosis, hyperkeratosis, and scaling.2 Of more than 20 ichthyoses, XLI is the second most common ichthyosis, with a prevalence of 1 in 6000 males.3 X-linked ichthyosis occurs almost exclusively in males, and although females can be carriers, they rarely exhibit skin manifestations.4
X-linked ichthyosis is caused by either a partial or full deletion or mutation in the STS gene on the X chromosome.2 The absence of STS activity results in the accumulation of cholesterol sulfate in the stratum corneum, leading to corneocyte cohesion, hyperkeratosis, and impaired skin permeability. The most common clinical phenotype is characterized by polygonal scales concentrated on the upper and lower extremities as well as the trunk (Figure), consistent with our patient's clinical presentation.5

anterior knees (A) as well as large exophytic papules on the upper
chest and neck (B).
X-linked ichthyosis typically presents in the first 6 months of life as generalized desquamation and xerosis that progresses to fine scaling on the trunk and extremities, more commonly and heavily involving the legs; however, the extensor surfaces of the arms also may be affected.6 After the neonatal period, fine scaling persists on the trunk and extremities, but scales often become coarser and darker over time. Although scaling is generalized, it typically spares the antecubital and popliteal fossae, palms, soles, and midface. The lateral face, axillae, and neck always remain involved.4 The most common extracutaneous manifestations of XLI affect the ocular, genitourinary, and cognitive/behavioral systems. Patients can develop corneal comma-shaped opacities, hypogonadism, cryptorchidism, and an increased risk for testicular cancer. Female carriers may have prolonged delivery of affected neonates.2,5,7-9 Given the unrelated debilitating neurologic consequences of our patient's presenting subarachnoid hemorrhage, further workup was not pursued into these associations.
Although XLI is most commonly diagnosed in early childhood, it also must be considered in adult patients presenting with severe scaling of the trunk, arms, and legs who have not had prior dermatologic workup. Given the similarity of XLI presentation to other ichthyoses, particularly ichthyosis vulgaris, lamellar ichthyosis, and ichthyosis bullosa of Siemens, genetic analysis is the most accurate diagnostic tool and should be considered in patients with an atypical presentation. Rupioid psoriasis also may be considered and can be confirmed on biopsy. Diagnosis of XLI should prompt symptomatic treatment, genetic counseling, and workup for extracutaneous manifestations.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
- Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol. 1965;92:1-6.
- Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485.
- Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141:617-627.
- Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis [published online November 27, 2013]. Biochim Biophys Acta. 2014;1841:353-361.
- Wu B, Paller AS. Ichthyosis, X-Linked. Treasure Island, FL: StatPearls Publishing LLC; 2019.
- Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res. 2016;5. doi:10.12688/f1000research.8584.1.
- Baek WS, Aypar U. Case report neurological manifestations of X-linked ichthyosis: case report and review of the literature [published online August 13, 2017]. 2017;2017:9086408.
- Brookes KJ, Hawi Z, Park J, et al. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:1417-1424.
- Kent L, Emerton J, Bhadravathi V, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524.
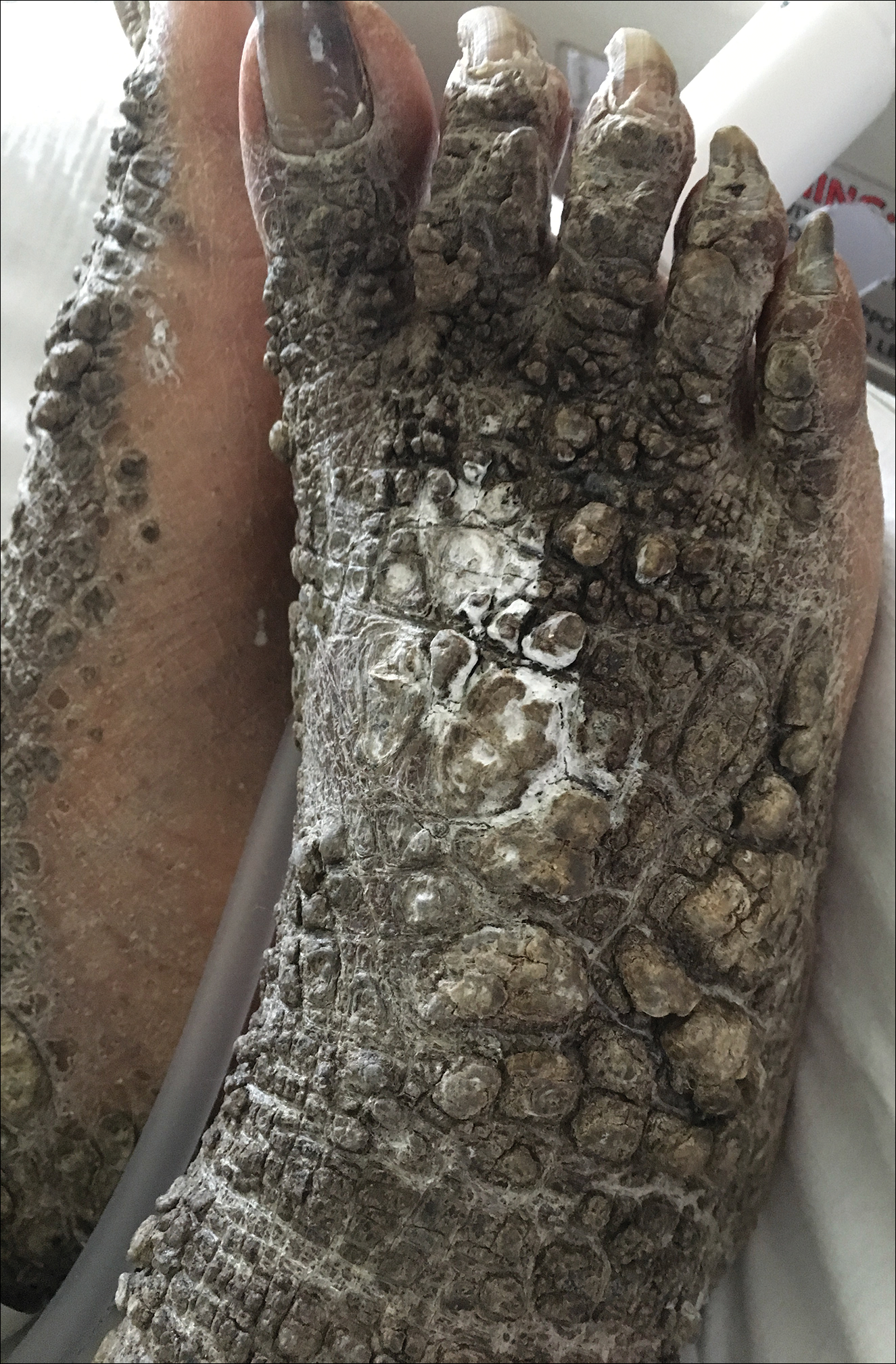
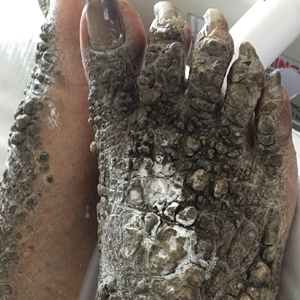
A 67-year-old man with a history of congestive heart failure, type 2 diabetes mellitus, hypertension, and schizophrenia was admitted to the hospital for subarachnoid hemorrhage and was noted to have heavy scaling on the bilateral legs. Given recent medical events, the patient was nonconversant at the time of consultation, but his daughter provided his medical history at bedside. The patient usually wore long-sleeved clothing and pants, thus no one had seen his skin in many years, and it was unclear how long the scaling had been present. His family history was notable for eczema in distant relatives but negative for comparable conditions. Physical examination revealed thick lichenified skin with many large, exophytic, brown papules (largest measured 1.5×1×1 cm) and platelike scaling on the anterior chest, abdomen, lateral arms, and forearms. Extensive coalescing hyperkeratotic plaques and papules (up to 1 cm in thickness) were present on the anterior legs and feet, and scattered verrucous brown papules were noted on the plantar aspects of the bilateral feet. A punch biopsy of the left foot revealed extensive, dense, compact, orthokeratotic hyperkeratosis with a preserved granular layer with no epidermolysis.