User login
Strategies and steps for the surgical management of endometriosis
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).
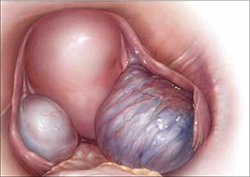
FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.
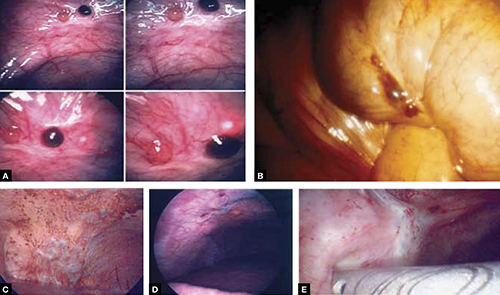
FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?
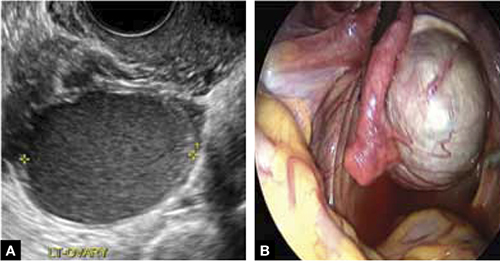
FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.
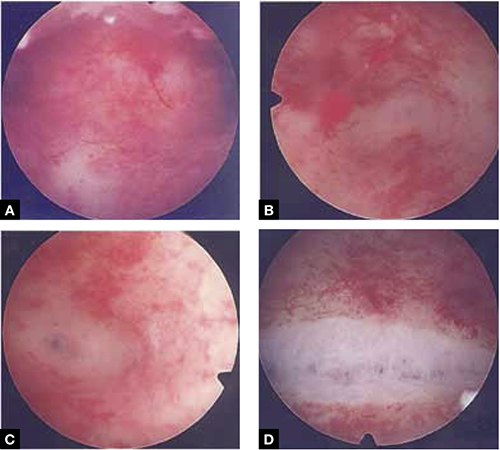
FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.
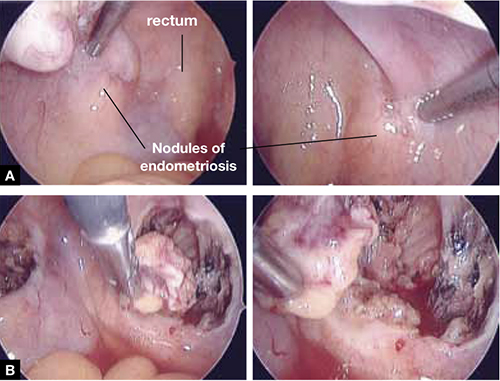
FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.
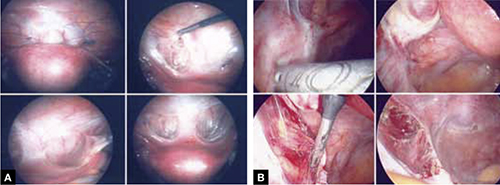
FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).

FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.

FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?

FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.

FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.

FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.

FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).

FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.

FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?

FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.

FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.

FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.

FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.
Q.Does time since menopause determine how HRT affects cardiovascular health?
Expert Commentary
The putative protective effects of HRT on the risk of cardiovascular disease (CVD) suggested by observational studies for many years1 were completely negated by prospective, randomized trials.2,3 The WHI clinical trials reported no benefits with unopposed estrogen and a statistically significant greater risk of CVD events with combination HRT (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.00–1.54).
The divergent results between observational studies and clinical trials have been attributed to several potential confounding factors, including methodologic differences such as healthy-use bias, compliance bias, and incomplete capture of early clinical events, or biological differences such as formulation and dose of the hormone regimen, time since menopause, and stage of atherosclerosis.4
Interestingly, observational studies of HRT in menopausal women have been remarkably consistent with randomized studies in predicting other risks, such as stroke, breast cancer, and thromboembolic events, as well as the benefits associated with HRT in regard to osteoporosis-related fractures and colon cancer. There is apparently something unique about CHD that accounts for divergent results between observational and controlled studies.
Earlier data suggested HRT is better suited to younger women
Rossouw and colleagues address 2 confounding factors—years since menopause and age of subjects when they started HRT—to explore the possibility that HRT may protect against CVD in younger, healthier women and be hazardous in older women who have preexisting cardiovascular disease. Support for this hypothesis comes from several sources, including animal studies and controlled and observational studies in postmenopausal women.
For example, in observational studies such as the Nurses Health Study, which consistently reported HRT-related protective effects on CVD, the postmenopausal women were younger (between 30 and 55 years old) and leaner (mean body mass index [BMI], 24.3) and had begun using hormones within 2 years after menopause.5 They were, overall, quite different from the menopausal women in the WHI, who were older (mean age, 63 years) and heavier (mean BMI of 28.5) and who had been menopausal for about 10 years at the time of enrollment, when they started using HRT.2
Findings confirm greater hazard for women well past menopause
Rossouw and colleagues conducted secondary analyses of data from the 2 WHI randomized trials, looking at the effect of HRT on CHD and stroke across categories of age and years since menopause. They found that:
- Among younger women with less than 10 years since menopause, the hazard ratio (HR) for CHD was 0.76 (CI, 0.50–1.16), compared with 1.10 (CI, 0.84–1.45) and 1.28 (CI, 1.03–1.58) for the older groups with, respectively, 10 to 19 and more than 20 years after menopause
- The effects of HRT on total mortality tended to be more favorable in younger women than in older women (P for trend, .06)
- The presence of vasomotor symptoms at baseline had a significant impact on the increased risk of CHD with HRT in women age 70 to 79 years or in women with 20 or more years since menopause—but not in the younger group
- HRT increased the risk of stroke by 32%, regardless of age and years since menopause.
In younger women, hormones are a reasonable, short-term option
These secondary analyses of WHI data help us understand the divergent results between observational and controlled studies on the effects of HRT on CHD risk in postmenopausal women, and confirm the hypothesis that the health consequences of HRT may vary by distance from menopause, being absent in women close to menopause but significantly high in women distant from menopause, especially if they have vasomotor symptoms.
These data offer some reassurance that, in younger women, hormones remain a reasonable option for short-term treatment of menopausal symptoms but do not necessarily imply an absence of harm, especially over prolonged use.
Limitations of the trial
Although Rossouw and colleagues explore 2 important confounding variables, they did not address others, such as characteristics of study populations (such as estrogen levels) or different hormone regimens, which may be equally, if not more, important in determining the risk–benefit ratio of HRT in menopausal women. It is possible that women who have a lower BMI and who have a lower level of endogenous estrogen may constitute a group that benefits uniquely from hormone use, as a large cohort study of 290,827 postmenopausal women has suggested.6
It also may be that a different progestin may further reduce the CHD risk by inducing a better lipid profile, reducing plaque formation, and diminishing coronary artery reactivity and blood flow.
Clinical recommendation
These new data do not alter the current recommendation that HRT be used for the relief of disturbing vasomotor symptoms at the lowest effective dose and for the shortest tolerable time.7
However, we still have much to learn about the use of hormones in postmenopausal women, and need additional studies designed to allow us to develop the hormone regimen with the best safety and efficacy profile, which should be applied to the subgroups of postmenopausal women that will derive the most benefit.
1. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605-613.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on menopausal hormone therapy. N Engl J Med. 2003;349:645-650.
5. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
6. Rodriguez C, Calle EE, Patel AV, Tatham AM, et al. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153:145-152.
7. ACOG Task Force on Hormone Therapy. Hormone Therapy. Obstet Gynecol. 2004;104(Suppl):1S-131S.
Expert Commentary
The putative protective effects of HRT on the risk of cardiovascular disease (CVD) suggested by observational studies for many years1 were completely negated by prospective, randomized trials.2,3 The WHI clinical trials reported no benefits with unopposed estrogen and a statistically significant greater risk of CVD events with combination HRT (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.00–1.54).
The divergent results between observational studies and clinical trials have been attributed to several potential confounding factors, including methodologic differences such as healthy-use bias, compliance bias, and incomplete capture of early clinical events, or biological differences such as formulation and dose of the hormone regimen, time since menopause, and stage of atherosclerosis.4
Interestingly, observational studies of HRT in menopausal women have been remarkably consistent with randomized studies in predicting other risks, such as stroke, breast cancer, and thromboembolic events, as well as the benefits associated with HRT in regard to osteoporosis-related fractures and colon cancer. There is apparently something unique about CHD that accounts for divergent results between observational and controlled studies.
Earlier data suggested HRT is better suited to younger women
Rossouw and colleagues address 2 confounding factors—years since menopause and age of subjects when they started HRT—to explore the possibility that HRT may protect against CVD in younger, healthier women and be hazardous in older women who have preexisting cardiovascular disease. Support for this hypothesis comes from several sources, including animal studies and controlled and observational studies in postmenopausal women.
For example, in observational studies such as the Nurses Health Study, which consistently reported HRT-related protective effects on CVD, the postmenopausal women were younger (between 30 and 55 years old) and leaner (mean body mass index [BMI], 24.3) and had begun using hormones within 2 years after menopause.5 They were, overall, quite different from the menopausal women in the WHI, who were older (mean age, 63 years) and heavier (mean BMI of 28.5) and who had been menopausal for about 10 years at the time of enrollment, when they started using HRT.2
Findings confirm greater hazard for women well past menopause
Rossouw and colleagues conducted secondary analyses of data from the 2 WHI randomized trials, looking at the effect of HRT on CHD and stroke across categories of age and years since menopause. They found that:
- Among younger women with less than 10 years since menopause, the hazard ratio (HR) for CHD was 0.76 (CI, 0.50–1.16), compared with 1.10 (CI, 0.84–1.45) and 1.28 (CI, 1.03–1.58) for the older groups with, respectively, 10 to 19 and more than 20 years after menopause
- The effects of HRT on total mortality tended to be more favorable in younger women than in older women (P for trend, .06)
- The presence of vasomotor symptoms at baseline had a significant impact on the increased risk of CHD with HRT in women age 70 to 79 years or in women with 20 or more years since menopause—but not in the younger group
- HRT increased the risk of stroke by 32%, regardless of age and years since menopause.
In younger women, hormones are a reasonable, short-term option
These secondary analyses of WHI data help us understand the divergent results between observational and controlled studies on the effects of HRT on CHD risk in postmenopausal women, and confirm the hypothesis that the health consequences of HRT may vary by distance from menopause, being absent in women close to menopause but significantly high in women distant from menopause, especially if they have vasomotor symptoms.
These data offer some reassurance that, in younger women, hormones remain a reasonable option for short-term treatment of menopausal symptoms but do not necessarily imply an absence of harm, especially over prolonged use.
Limitations of the trial
Although Rossouw and colleagues explore 2 important confounding variables, they did not address others, such as characteristics of study populations (such as estrogen levels) or different hormone regimens, which may be equally, if not more, important in determining the risk–benefit ratio of HRT in menopausal women. It is possible that women who have a lower BMI and who have a lower level of endogenous estrogen may constitute a group that benefits uniquely from hormone use, as a large cohort study of 290,827 postmenopausal women has suggested.6
It also may be that a different progestin may further reduce the CHD risk by inducing a better lipid profile, reducing plaque formation, and diminishing coronary artery reactivity and blood flow.
Clinical recommendation
These new data do not alter the current recommendation that HRT be used for the relief of disturbing vasomotor symptoms at the lowest effective dose and for the shortest tolerable time.7
However, we still have much to learn about the use of hormones in postmenopausal women, and need additional studies designed to allow us to develop the hormone regimen with the best safety and efficacy profile, which should be applied to the subgroups of postmenopausal women that will derive the most benefit.
Expert Commentary
The putative protective effects of HRT on the risk of cardiovascular disease (CVD) suggested by observational studies for many years1 were completely negated by prospective, randomized trials.2,3 The WHI clinical trials reported no benefits with unopposed estrogen and a statistically significant greater risk of CVD events with combination HRT (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.00–1.54).
The divergent results between observational studies and clinical trials have been attributed to several potential confounding factors, including methodologic differences such as healthy-use bias, compliance bias, and incomplete capture of early clinical events, or biological differences such as formulation and dose of the hormone regimen, time since menopause, and stage of atherosclerosis.4
Interestingly, observational studies of HRT in menopausal women have been remarkably consistent with randomized studies in predicting other risks, such as stroke, breast cancer, and thromboembolic events, as well as the benefits associated with HRT in regard to osteoporosis-related fractures and colon cancer. There is apparently something unique about CHD that accounts for divergent results between observational and controlled studies.
Earlier data suggested HRT is better suited to younger women
Rossouw and colleagues address 2 confounding factors—years since menopause and age of subjects when they started HRT—to explore the possibility that HRT may protect against CVD in younger, healthier women and be hazardous in older women who have preexisting cardiovascular disease. Support for this hypothesis comes from several sources, including animal studies and controlled and observational studies in postmenopausal women.
For example, in observational studies such as the Nurses Health Study, which consistently reported HRT-related protective effects on CVD, the postmenopausal women were younger (between 30 and 55 years old) and leaner (mean body mass index [BMI], 24.3) and had begun using hormones within 2 years after menopause.5 They were, overall, quite different from the menopausal women in the WHI, who were older (mean age, 63 years) and heavier (mean BMI of 28.5) and who had been menopausal for about 10 years at the time of enrollment, when they started using HRT.2
Findings confirm greater hazard for women well past menopause
Rossouw and colleagues conducted secondary analyses of data from the 2 WHI randomized trials, looking at the effect of HRT on CHD and stroke across categories of age and years since menopause. They found that:
- Among younger women with less than 10 years since menopause, the hazard ratio (HR) for CHD was 0.76 (CI, 0.50–1.16), compared with 1.10 (CI, 0.84–1.45) and 1.28 (CI, 1.03–1.58) for the older groups with, respectively, 10 to 19 and more than 20 years after menopause
- The effects of HRT on total mortality tended to be more favorable in younger women than in older women (P for trend, .06)
- The presence of vasomotor symptoms at baseline had a significant impact on the increased risk of CHD with HRT in women age 70 to 79 years or in women with 20 or more years since menopause—but not in the younger group
- HRT increased the risk of stroke by 32%, regardless of age and years since menopause.
In younger women, hormones are a reasonable, short-term option
These secondary analyses of WHI data help us understand the divergent results between observational and controlled studies on the effects of HRT on CHD risk in postmenopausal women, and confirm the hypothesis that the health consequences of HRT may vary by distance from menopause, being absent in women close to menopause but significantly high in women distant from menopause, especially if they have vasomotor symptoms.
These data offer some reassurance that, in younger women, hormones remain a reasonable option for short-term treatment of menopausal symptoms but do not necessarily imply an absence of harm, especially over prolonged use.
Limitations of the trial
Although Rossouw and colleagues explore 2 important confounding variables, they did not address others, such as characteristics of study populations (such as estrogen levels) or different hormone regimens, which may be equally, if not more, important in determining the risk–benefit ratio of HRT in menopausal women. It is possible that women who have a lower BMI and who have a lower level of endogenous estrogen may constitute a group that benefits uniquely from hormone use, as a large cohort study of 290,827 postmenopausal women has suggested.6
It also may be that a different progestin may further reduce the CHD risk by inducing a better lipid profile, reducing plaque formation, and diminishing coronary artery reactivity and blood flow.
Clinical recommendation
These new data do not alter the current recommendation that HRT be used for the relief of disturbing vasomotor symptoms at the lowest effective dose and for the shortest tolerable time.7
However, we still have much to learn about the use of hormones in postmenopausal women, and need additional studies designed to allow us to develop the hormone regimen with the best safety and efficacy profile, which should be applied to the subgroups of postmenopausal women that will derive the most benefit.
1. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605-613.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on menopausal hormone therapy. N Engl J Med. 2003;349:645-650.
5. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
6. Rodriguez C, Calle EE, Patel AV, Tatham AM, et al. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153:145-152.
7. ACOG Task Force on Hormone Therapy. Hormone Therapy. Obstet Gynecol. 2004;104(Suppl):1S-131S.
1. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605-613.
3. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
4. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on menopausal hormone therapy. N Engl J Med. 2003;349:645-650.
5. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
6. Rodriguez C, Calle EE, Patel AV, Tatham AM, et al. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153:145-152.
7. ACOG Task Force on Hormone Therapy. Hormone Therapy. Obstet Gynecol. 2004;104(Suppl):1S-131S.
Q Which works better: Vaginal or oral estrogen for atrophy and dyspareunia?
Expert Commentary
METHODS Investigators randomized 57 postmenopausal, hysterectomized women to receive oral (0.625 mg of conjugated equine estrogen [CEE]; n=27) or vaginal (0.625 mg of CEE per 1 g of vaginal cream; n=30) estrogen once daily.
Vaginal vascularization and sexual function were assessed through a variety of measures, including serum estradiol, introital color Doppler ultrasound, and personal interviews.
RESULTS After 3 months of treatment, both groups of women had significant increases in the number of vaginal vessels, and “marked” decreases in the pulsatility index.
Unopposed estrogen has its own benefits, risks
In hysterectomized postmenopausal women, unopposed estrogen has been shown to relieve menopausal symptoms and protect against bone loss and osteoporosis-related fractures without increasing the risk of breast cancer or cardiovascular disease.
Don’t be fooled by serum levels
Measurements of serum estradiol levels in women who are taking CEE either orally or vaginally do not truly reflect the total estrogenic load of these patients, because the bulk of estrogen in CEE is estrone sulfate with several equine estrogenic compounds that have activity not reflected by serum estradiol. Consequently, the estrogenicity of the women in this study was not accurately evaluated through the serum estradiol measurements.
Vaginal estrogen is not “topical”
Moreover, the authors refer to the vaginal group as receiving “topical” estrogen—another misconception. Systemic absorption of vaginal estrogen is probably greater and more rapid than with the oral route. It is no surprise that vaginal administration was associated with greater improvement of both dyspareunia and vaginal dryness, because the vagina is exposed to a greater concentration of estrogen when it is administered vaginally than when it is given orally. With oral administration, a significant amount of estrogen is metabolized by the liver, and a much lower dose of estrogen reaches the vaginal epithelium.
Oral estrogen can reduce libido
The lack of improved libido with the oral preparation is not surprising. In fact, some women may experience a decrease in libido with orally administered estrogen because of the associated increases in SHBG. With vaginal administration, SHBG levels are not increased, and sexual desire may improve, especially when vaginal lubrication is improved.
Does either form affect sexual function? Unfortunately, this study was not long enough or sufficiently powered to adequately assess sexual function.
Systemic absorption is high even with vaginal route
The same dose of CEE yields slightly greater benefits to vaginal health and function when it is administered vaginally. When estrogen is given vaginally, systemic absorption is significant—and may be greater than with the oral route.
Indications and contraindications of oral estrogen, consequently, also apply to vaginal administration.
1. Scarabin PY, Oger E, Plu-Bureau G. For the Estrogen and Thromboembolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
2. Straczek C, Oger E, Yon de Jonage-Canonico MB, et al. For the Estrogen and Thromboembolism Risk Study Group. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation. 2005;112:3495-3500.
Expert Commentary
METHODS Investigators randomized 57 postmenopausal, hysterectomized women to receive oral (0.625 mg of conjugated equine estrogen [CEE]; n=27) or vaginal (0.625 mg of CEE per 1 g of vaginal cream; n=30) estrogen once daily.
Vaginal vascularization and sexual function were assessed through a variety of measures, including serum estradiol, introital color Doppler ultrasound, and personal interviews.
RESULTS After 3 months of treatment, both groups of women had significant increases in the number of vaginal vessels, and “marked” decreases in the pulsatility index.
Unopposed estrogen has its own benefits, risks
In hysterectomized postmenopausal women, unopposed estrogen has been shown to relieve menopausal symptoms and protect against bone loss and osteoporosis-related fractures without increasing the risk of breast cancer or cardiovascular disease.
Don’t be fooled by serum levels
Measurements of serum estradiol levels in women who are taking CEE either orally or vaginally do not truly reflect the total estrogenic load of these patients, because the bulk of estrogen in CEE is estrone sulfate with several equine estrogenic compounds that have activity not reflected by serum estradiol. Consequently, the estrogenicity of the women in this study was not accurately evaluated through the serum estradiol measurements.
Vaginal estrogen is not “topical”
Moreover, the authors refer to the vaginal group as receiving “topical” estrogen—another misconception. Systemic absorption of vaginal estrogen is probably greater and more rapid than with the oral route. It is no surprise that vaginal administration was associated with greater improvement of both dyspareunia and vaginal dryness, because the vagina is exposed to a greater concentration of estrogen when it is administered vaginally than when it is given orally. With oral administration, a significant amount of estrogen is metabolized by the liver, and a much lower dose of estrogen reaches the vaginal epithelium.
Oral estrogen can reduce libido
The lack of improved libido with the oral preparation is not surprising. In fact, some women may experience a decrease in libido with orally administered estrogen because of the associated increases in SHBG. With vaginal administration, SHBG levels are not increased, and sexual desire may improve, especially when vaginal lubrication is improved.
Does either form affect sexual function? Unfortunately, this study was not long enough or sufficiently powered to adequately assess sexual function.
Systemic absorption is high even with vaginal route
The same dose of CEE yields slightly greater benefits to vaginal health and function when it is administered vaginally. When estrogen is given vaginally, systemic absorption is significant—and may be greater than with the oral route.
Indications and contraindications of oral estrogen, consequently, also apply to vaginal administration.
Expert Commentary
METHODS Investigators randomized 57 postmenopausal, hysterectomized women to receive oral (0.625 mg of conjugated equine estrogen [CEE]; n=27) or vaginal (0.625 mg of CEE per 1 g of vaginal cream; n=30) estrogen once daily.
Vaginal vascularization and sexual function were assessed through a variety of measures, including serum estradiol, introital color Doppler ultrasound, and personal interviews.
RESULTS After 3 months of treatment, both groups of women had significant increases in the number of vaginal vessels, and “marked” decreases in the pulsatility index.
Unopposed estrogen has its own benefits, risks
In hysterectomized postmenopausal women, unopposed estrogen has been shown to relieve menopausal symptoms and protect against bone loss and osteoporosis-related fractures without increasing the risk of breast cancer or cardiovascular disease.
Don’t be fooled by serum levels
Measurements of serum estradiol levels in women who are taking CEE either orally or vaginally do not truly reflect the total estrogenic load of these patients, because the bulk of estrogen in CEE is estrone sulfate with several equine estrogenic compounds that have activity not reflected by serum estradiol. Consequently, the estrogenicity of the women in this study was not accurately evaluated through the serum estradiol measurements.
Vaginal estrogen is not “topical”
Moreover, the authors refer to the vaginal group as receiving “topical” estrogen—another misconception. Systemic absorption of vaginal estrogen is probably greater and more rapid than with the oral route. It is no surprise that vaginal administration was associated with greater improvement of both dyspareunia and vaginal dryness, because the vagina is exposed to a greater concentration of estrogen when it is administered vaginally than when it is given orally. With oral administration, a significant amount of estrogen is metabolized by the liver, and a much lower dose of estrogen reaches the vaginal epithelium.
Oral estrogen can reduce libido
The lack of improved libido with the oral preparation is not surprising. In fact, some women may experience a decrease in libido with orally administered estrogen because of the associated increases in SHBG. With vaginal administration, SHBG levels are not increased, and sexual desire may improve, especially when vaginal lubrication is improved.
Does either form affect sexual function? Unfortunately, this study was not long enough or sufficiently powered to adequately assess sexual function.
Systemic absorption is high even with vaginal route
The same dose of CEE yields slightly greater benefits to vaginal health and function when it is administered vaginally. When estrogen is given vaginally, systemic absorption is significant—and may be greater than with the oral route.
Indications and contraindications of oral estrogen, consequently, also apply to vaginal administration.
1. Scarabin PY, Oger E, Plu-Bureau G. For the Estrogen and Thromboembolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
2. Straczek C, Oger E, Yon de Jonage-Canonico MB, et al. For the Estrogen and Thromboembolism Risk Study Group. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation. 2005;112:3495-3500.
1. Scarabin PY, Oger E, Plu-Bureau G. For the Estrogen and Thromboembolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
2. Straczek C, Oger E, Yon de Jonage-Canonico MB, et al. For the Estrogen and Thromboembolism Risk Study Group. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation. 2005;112:3495-3500.
Q Does ultralow-dose, transdermal estradiol prevent bone loss?
<huc>A</huc> Yes, but benefits and risks should be weighed for each patient in comparison to other osteoporosis preventives, and the effect on vasomotor symptoms is unknown.
Expert Commentary
In this excellent prospective, randomized trial, Ettinger et al seem to have defined the lowest effective dose of transdermal estradiol to preserve bone mineral density in women 60 to 80 years of age. During 2 years of treatment, a daily dose of .014 mg estradiol was more effective than placebo in increasing bone density of both spine and hip. It also caused a significant decrease in markers of bone turnover. And though the estradiol was unopposed, risks of vaginal bleeding and endometrial hyperplasia did not rise.
Too brief to show effect on fractures
The 2 years of study were, understandably, not enough to show a reduction in fractures. Nor does the study show whether this therapy relieves menopausal symptoms, the only other approved indications for estrogen or estrogen-progestin therapy in menopausal women. This, too, is understandable, since the study focused on women well past menopause.
Estrogen’s risks: Still much to learn
More important is the fact that the potential risks of estrogen and estrogen-progestin therapy have not been fully clarified. Although the risk of endometrial hyperplasia did not increase in 2 years of study, might it rise after 3 or 4 more years? And will the risks of breast cancer, thromboembolic events, or stroke increase with ultralow-dose, transdermal estradiol, as they did with conventional doses of oral conjugated equine estrogen in 1 or both arms of the Women’s Health Initiative?
Prescribe for osteoporosis prevention, not menopausal symptoms
Over 2 years, .014 mg daily of transdermal estradiol prevents bone loss without increasing endometrial hyperplasia, but the lack of data on menopausal symptoms limits the drug’s applicability.
Ultralow-dose estrogen patches should be prescribed only for prevention of osteoporosis after consideration of other options, such as calcium, vitamin D, raloxifene, and bisphosphonates. Physicians should keep in mind the Food and Drug Administration’s directive to prescribe estrogen at the lowest effective dose for the shortest time possible.
The author has received grant and research support from Berlex, Solvay, and Procter & Gamble, and serves on the speakers bureau for Eli Lilly, Procter & Gamble, and Wyeth.
<huc>A</huc> Yes, but benefits and risks should be weighed for each patient in comparison to other osteoporosis preventives, and the effect on vasomotor symptoms is unknown.
Expert Commentary
In this excellent prospective, randomized trial, Ettinger et al seem to have defined the lowest effective dose of transdermal estradiol to preserve bone mineral density in women 60 to 80 years of age. During 2 years of treatment, a daily dose of .014 mg estradiol was more effective than placebo in increasing bone density of both spine and hip. It also caused a significant decrease in markers of bone turnover. And though the estradiol was unopposed, risks of vaginal bleeding and endometrial hyperplasia did not rise.
Too brief to show effect on fractures
The 2 years of study were, understandably, not enough to show a reduction in fractures. Nor does the study show whether this therapy relieves menopausal symptoms, the only other approved indications for estrogen or estrogen-progestin therapy in menopausal women. This, too, is understandable, since the study focused on women well past menopause.
Estrogen’s risks: Still much to learn
More important is the fact that the potential risks of estrogen and estrogen-progestin therapy have not been fully clarified. Although the risk of endometrial hyperplasia did not increase in 2 years of study, might it rise after 3 or 4 more years? And will the risks of breast cancer, thromboembolic events, or stroke increase with ultralow-dose, transdermal estradiol, as they did with conventional doses of oral conjugated equine estrogen in 1 or both arms of the Women’s Health Initiative?
Prescribe for osteoporosis prevention, not menopausal symptoms
Over 2 years, .014 mg daily of transdermal estradiol prevents bone loss without increasing endometrial hyperplasia, but the lack of data on menopausal symptoms limits the drug’s applicability.
Ultralow-dose estrogen patches should be prescribed only for prevention of osteoporosis after consideration of other options, such as calcium, vitamin D, raloxifene, and bisphosphonates. Physicians should keep in mind the Food and Drug Administration’s directive to prescribe estrogen at the lowest effective dose for the shortest time possible.
The author has received grant and research support from Berlex, Solvay, and Procter & Gamble, and serves on the speakers bureau for Eli Lilly, Procter & Gamble, and Wyeth.
<huc>A</huc> Yes, but benefits and risks should be weighed for each patient in comparison to other osteoporosis preventives, and the effect on vasomotor symptoms is unknown.
Expert Commentary
In this excellent prospective, randomized trial, Ettinger et al seem to have defined the lowest effective dose of transdermal estradiol to preserve bone mineral density in women 60 to 80 years of age. During 2 years of treatment, a daily dose of .014 mg estradiol was more effective than placebo in increasing bone density of both spine and hip. It also caused a significant decrease in markers of bone turnover. And though the estradiol was unopposed, risks of vaginal bleeding and endometrial hyperplasia did not rise.
Too brief to show effect on fractures
The 2 years of study were, understandably, not enough to show a reduction in fractures. Nor does the study show whether this therapy relieves menopausal symptoms, the only other approved indications for estrogen or estrogen-progestin therapy in menopausal women. This, too, is understandable, since the study focused on women well past menopause.
Estrogen’s risks: Still much to learn
More important is the fact that the potential risks of estrogen and estrogen-progestin therapy have not been fully clarified. Although the risk of endometrial hyperplasia did not increase in 2 years of study, might it rise after 3 or 4 more years? And will the risks of breast cancer, thromboembolic events, or stroke increase with ultralow-dose, transdermal estradiol, as they did with conventional doses of oral conjugated equine estrogen in 1 or both arms of the Women’s Health Initiative?
Prescribe for osteoporosis prevention, not menopausal symptoms
Over 2 years, .014 mg daily of transdermal estradiol prevents bone loss without increasing endometrial hyperplasia, but the lack of data on menopausal symptoms limits the drug’s applicability.
Ultralow-dose estrogen patches should be prescribed only for prevention of osteoporosis after consideration of other options, such as calcium, vitamin D, raloxifene, and bisphosphonates. Physicians should keep in mind the Food and Drug Administration’s directive to prescribe estrogen at the lowest effective dose for the shortest time possible.
The author has received grant and research support from Berlex, Solvay, and Procter & Gamble, and serves on the speakers bureau for Eli Lilly, Procter & Gamble, and Wyeth.
From the Women’s Health Initiative to clinical practice: A 5-point plan
- Give estrogen and estrogen-progestin therapy only for the relief of significant vasomotor symptoms, and halt the therapy in all asymptomatic women.
- Prescribe natural estrogens and progesterone whenever possible and measure serum levels to assess response and compliance.
- Initiate therapy at a dose of 0.3 mg for conjugated equine estrogen, 0.5 mg for oral estradiol, and 0.035 mg for transdermal estradiol and progressively increase, if necessary, to no more than twice these amounts.
- Give progesterone cyclically rather than continuously to reduce risk of cardiovascular disease, breast cancer, other adverse events.
- Reassess regimens annually in each patient.
I replied that most of my symptomatic postmenopausal patients continued taking estrogen therapy (ET) or EPT. Still, I understood the concern that prompted the question.
After the WHI findings became widely publicized, many women discontinued EPT—only to resume therapy 8 to 10 weeks later because of persistent vasomotor symptoms. A number of other postmenopausal women, however, were able to cope with the transient recurrence of menopausal symptoms. I was happy to encourage them to permanently discontinue ET/EPT, since the primary reason for starting the therapy (vasomotor symptoms) was no longer an issue and the secondary reason (long-term benefits originally attributed to therapy with estrogen and progestin) had been seriously challenged.
This article discusses the American College of Obstetricians and Gynecologists guidelines on ET/EPT,2 and includes 5 specific pointers on managing menopausal women at risk for osteoporosis, breast cancer, and cardiovascular disease.
The bottom line: The WHI has significantly affected clinical practice, but hormone use is not precluded in symptomatic post-menopausal women, whose distressing symptoms and quality of life are improved by EPT.
TABLE 1
Relative risk of selected adverse events in women taking estrogen-progestin therapy1
| EVENT | RELATIVE RISK | CONFIDENCE INTERVAL | STATISTICAL SIGNIFICANCE |
|---|---|---|---|
| Coronary heart disease | 1.29 | 1.02–1.63 | Yes |
| Stroke | 1.41 | 1.07–1.85 | Yes |
| Breast cancer | 1.26 | 1.00–1.59 | Yes |
| Pulmonary embolism | 2.13 | 1.39–3.25 | Yes |
| Endometrial cancer | 0.83 | 0.47–1.47 | No |
| Hip fracture | 0.66 | 0.45–0.98 | Yes |
| Colorectal cancer | 0.63 | 0.43–0.92 | Yes |
| Deep venous thrombosis | 2.07 | 1.49–2.87 | Yes |
| Vertebral fractures | 0.66 | 0.44–0.98 | Yes |
| Other osteoporotic fractures | 0.77 | 0.69–0.86 | Yes |
| Global index | 1.15 | 1.03–1.28 | Yes |
TABLE 2
Number of selected adverse events per 10,000 woman-years1*
| EVENT | PLACEBO (N = 8,102) | CEE/MPA (N=8,506) | DELTA |
|---|---|---|---|
| Coronary heart disease | 30 | 37 | 7 |
| Stroke | 21 | 29 | 9 |
| Breast cancer | 30 | 38 | 8 |
| Pulmonary embolism | 8 | 16 | 8 |
| Endometrial cancer | 6 | 5 | -1 |
| Hip fracture | 15 | 10 | -5 |
| Colorectal cancer | 16 | 10 | -6 |
| Deep venous thrombosis† | 3 | 26 | 16 |
| Vertebral fractures† | 15 | 9 | -6 |
| Other osteoporotic fractures† | 170 | 131 | -49 |
| Global index | 151 | 170 | 19 |
| CEE = conjugated equine estrogen | |||
| MPA = medroxyprogesterone acetate | |||
| *Absolute risk | |||
| †Not included in global index | |||
1. Individualize treatment
In the realm of estrogen therapy, 1 size does not fit all. Women tolerate and respond differently to various preparations and doses. I tend to use estradiol and micronized progesterone as much as possible because I believe they may be safer. With natural hormones it also is easier to measure serum levels; this helps me assess a patient’s response to and compliance with therapy.
I prefer the transdermal preparation consisting of estradiol/norethindrone acetate (CombiPatch) because it is more physiologic and yields constant serum levels throughout the 24-hour day. In addition, it avoids the first pass through the liver, which is associated with nonphysiologic alteration of hepatic enzymes and proteins—especially binding globulins for steroids, thyroid and sex hormones, and coagulation factors.
2. Use the lowest effective dose to achieve benefits and avoid side effects
This is a critical recommendation. Although this rule should apply to all therapies, it is especially important for ET/EPT, which can be associated with life-threatening side effects.
In a prospective, observational study conducted in healthy postmenopausal women, Grodstein et al3 explored primary prevention of cardiovascular disease. They found that the relative risk of stroke rose with each incremental increase in the dose of unopposed conjugated equine estrogen (CEE). The relative risk of stroke with CEE at a daily dose of 0.3 mg, 0.625 mg, and 1.25 mg was 0.54 (95% confidence interval [CI], 0.28–1.06), 1.35 (95% CI, 1.08–1.68), and 1.63 (95% CI, 1.18–2.26), respectively. Thus, the lowest effective dose at initiation would be 0.3 mg for CEE. Comparable doses for oral estradiol and transdermal estradiol are 0.5 mg and 0.035 mg, respectively.
This dose may be progressively increased according to the patient’s response, but seldom should exceed double the starting dose. Similar guidelines should be followed for progestins.
3. Consider minimizing duration of progestin treatment
Prescribe ET for hysterectomized women and EPT for those with a uterus. This may seem like old advice, since all gynecologists know the importance of using EPT in women with a uterus. But the optimal formulation and administration of EPT remain elusive. It is now clear that not all progestins have the same physiologic effects—especially on cardiovascular health (see). For this reason, the type, sequence, dose, and duration of progestin administration should be carefully reevaluated.
Daily progestin is not physiologic. When it first became evident that unopposed estrogen causes endometrial cancer in women with a uterus, progestins were administered for 7, 10, or 12 days each month, usually in the form of medroxyprogesterone acetate (MPA), mimicking the cyclic production of progesterone by the premenopausal ovary. Although this regimen was effective in protecting against endometrial cancer, the cyclic administration of EPT induced undesirable uterine bleeding, which caused many women to stop the therapy. To avoid cyclic bleeding and improve compliance, continuous combined EPT preparations were introduced, which involved daily exposure to progestin—a level that is clearly not physiologic.
Because it appears from the WHI study that progestins may be mostly responsible for the increased risks of cardiovascular disease, stroke, and breast cancer—since the estrogen-alone arm of the trial continues—it may be prudent to minimize the duration of progestin treatment in postmenopausal women. Several studies have demonstrated that the cyclic administration of progestin for 2 weeks every 2 to 3 months may be as protective against endometrial cancer as monthly administration.4-6 Moreover, if they receive the lowest effective dose of daily estrogen (as outlined) and bi- or tri-monthly cyclic progesterone, most women will experience little or no bleeding.
Prior to the Women’s Health Initiative (WHI), estrogen therapy (ET) and estrogen-progestin therapy (EPT) were widely assumed to protect against cardiovascular disease. This assumption had been substantiated by epidemiologic studies, as well as experimental and clinical trials, which consistently reported that ET/EPT had beneficial effects on the lipid profile. Also noted were direct effects on vessel walls, evident in decreasing arteriosclerosis and increased vasodilatation.1 Were all these studies erroneous? Or is there an explanation for both pre- and post-WHI findings?
Observational studies are somewhat notorious for selection and compliance biases. Women who take hormones generally are healthier, better educated, and wealthier than nonusers; they also have fewer coronary risk factors. Thus, the favorable outcomes observed with ET and EPT may have simply reflected these women’s greater health at baseline. But I don’t think so.
Although these confounding factors may have exaggerated the benefits of ET/EPT, it is unlikely that they masked the increased risk observed with the WHI and other recently published prospective, randomized studies, such as the Heart and Estrogen/progestin Replacement Study (HERS)2 and the Women’s Estrogen for Stroke Trial (WEST).3 Moreover, the other benefits (osteoporosis and colon cancer protection) and risks (thromboembolic events and breast cancer) previously reported in observational studies have been appropriately confirmed by prospective, randomized studies. Only the cardiovascular benefits have not. Instead, they have been completely negated. Why?
There is no clear answer, but 1 explanation may be that the significant improvement in cardiovascular health care for women over the past 2 decades has obscured the cardiovascular benefits of EPT manifested 15 years ago in epidemiologic studies.
Indeed, 20 and 30 years ago, cardiovascular disease was considered a disease of men; very few women were evaluated or treated for hypertension or hyperlipidemia, 2 major cardiovascular disease risk factors. In women, ET/EPT may have been therapeutic, either indirectly by improving the lipid profile or directly by decreasing plaque formation and inducing vasodilatation through regulation of nitric oxide, prostaglandin synthetase, and membrane ionic permeability.1
During the past 15 years, women’s health care has changed greatly. Women who have hypertension or hyperlipidemia—especially those who participate in clinical trials2-4—are commonly treated with potent lipid-lowering agents and antihypertensive medications. These agents’ protective effects against cardiovascular disease would obscure any potential vascular benefits of ET/EPT. Consequently, only the adverse vascular effects of ET/EPT would be noted, since its potential benefits would have been usurped by the stronger cardiotropic statins and antihypertensive drugs.
Consider this corollary: Had the WHI patients at risk for fracture been treated with bisphosphonates, I doubt very much that the protective effects of ET/EPT on bone would have been noted. This hypothesis is supported by the recent Estrogen in the Prevention of Atherosclerosis Trial.5 In this prospective study, 222 post-menopausal women with low-density lipoprotein cholesterol levels in excess of 130 mg/dL were randomized to receive either 17-ß estradiol or placebo and were angiographically monitored for progression of coronary atherosclerosis.
The results revealed that the women randomized to 17-ß estradiol experienced significantly less progression of atherosclerosis than the placebo group. However, in those study patients who were also taking lipid-lowering therapy (statins), 17-ß estradiol did not confer additional benefits on coronary atherosclerosis.
It appears, then, that ET/EPT served its purpose for cardiovascular protection when there was a need for it—prior to the widespread use of cardioprotective agents. Now, with vastly improved women’s health care, ET/EPT for cardiovascular protection is neither needed nor safe.
Role of progestin. The fact that the estrogenonly arm of the WHI has not been interrupted supports the hypothesis that the progestin in the EPT preparation is mostly responsible for the increased risk of breast cancer and atherosclerosis, including coronary heart disease and stroke.
Moreover, medroxyprogesterone acetate (MPA)—the progestin used in the WHI—may be key to the cardiovascular risks6 observed in the trial. A different progestin, such as micronized progesterone or norethindrone acetate, may not confer similar risks.7 Indeed, the Postmenopausal Estrogen/Progestin Interventions study found that the addition of MPA to conjugated equine estrogen in postmenopausal women negated some of the beneficial effects of conjugated equine estrogen on the lipid profile, whereas the addition of micronized progesterone did not.8
Moreover, animal studies have found that MPA6—but not norethindrone acetate9 or micronized progesterone—negates the beneficial effects of estrogen on coronary plaque formation. Thus, it appears that different progestins have variable effects on atherosclerosis and that, among the various clinically available progestins, MPA may be particularly deleterious to cardiovascular health. Unfortunately, the results of these animal studies may not apply to humans. Still, they should serve as hypotheses for future prospective, randomized clinical trials.
Whether the WHI findings can be generalized to other hormone preparations is unclear. At present, the burden of proof for greater safety and efficacy lies with the sponsors of the other products.
REFERENCES
1. Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615-624.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
3. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
4. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
5. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. Ann Intern Med. 2001;135:939-953.
6. Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Artherioscler Thromb Vasc Biol. 1997;17:217-221.
7. Riis BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187:1101-1116.
8. Alexanderson P, Haarbo J, Sandholdt I, Shalmi M, Lawaetz H, Christiansen C. Norethindrone acetate enhances the antiatherogenic effect of 17-beta-estradiol: a secondary prevention study of aortic atherosclerosis in ovariectomized cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1998;18:902-907.
9. The Writing group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199-208.
4. Stop long-term, fixed-dose EPT in asymptomatic women
Also eliminate its use for cardiovascular protection. Asymptomatic women do not need—nor will they benefit from—ET/EPT, so there is no reason to use it. EPT’s potential benefit in terms of bone loss and fractures can be achieved with other agents that are FDA-approved for the prevention of osteoporosis but carry less risk of breast cancer, cardiovascular disease, or thromboembolic events.
During an interim analysis of the Women’s Health Initiative (WHI) estrogen-progestin arm, the data safety monitoring board determined that estrogen and progestin therapy (EPT) had a greater potential for harm than for benefit. So on July 9, 2002, after an average of 5.2 years of observation, that arm was halted.1
The other arm of the trial continued to examine the use of unopposed estrogen in hysterectomized women. The fact that it continues suggests that its global index is still not definitively unfavorable.
At the time the EPT arm was halted, its participants had an increased incidence of 26% for breast cancer, 41% for stroke, 29% for coronary heart disease events, and 110% for thromboembolic events, compared with the placebo group. On the benefit side, they experienced a 37% reduction in colorectal cancer and a 34% reduction in hip fractures. These reductions were not sufficient to offset the increased risk (TABLES 1 and 2). There was no difference in mortality rates between the EPT and placebo groups.
Absolute versus relative risk. Because relative risks are confusing and often misunderstood, the results were also reported in absolute values, expressed in the number of additional events for treated woman-years, which is more meaningful to individual women. Thus, among 10,000 women taking EPT for a year, there will be 8 more cases of invasive breast cancer, 8 more strokes, 8 more pulmonary embolic events, and 7 more myocardial infarctions, but 6 fewer cases of colorectal cancer and 5 fewer hip fractures.
The absolute risk for an individual woman remains quite small (less than 0.1% per year). When this risk was calculated for all events over the 5.2 years of the trial, approximately 100 more women in the hormone group experienced an adverse event than in the placebo group. If this figure is extrapolated to the population at large and to a longer treatment duration, the use of EPT could account for tens of thousands of additional adverse events.
Study excluded women with hot flashes. The WHI did not evaluate the relief of vasomotor symptoms. In fact, women with severe hot flashes were excluded from the study. This means that the major benefits and most common indication for EPT were not considered in the risk-benefit ratio. Had they been included, that ratio might have yielded different results.
Value of the data. Nevertheless, the WHI data do provide important information, which allows us to discuss the benefits and risks of EPT more precisely and more objectively, especially for asymptomatic postmenopausal women.
REFERENCE
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
- The bisphosphonates—alendronate and risedronate—are safe, effective, convenient (weekly dosing), and not associated with life-threatening side effects.
- Raloxifene, the selective estrogen receptor modulator, is another good alternative for prevention and treatment of osteoporosis in patients not at risk for thromboembolic events. Unlike ET/EPT, raloxifene is not associated with an increased risk of either breast cancer or cardiovascular events. In fact, data from the Multiple Outcomes of Raloxifene Evaluation study suggest that, besides protecting against bone loss and vertebral fractures,7 raloxifene may reduce the risk of breast cancer8 by as much as 75%.9 Nor was raloxifene found to increase the risk or incidence of cardiovascular disease.8 In fact, in a group of 1,035 postmenopausal women at high risk for cardiovascular disease, raloxifene reduced the incidence of all cardiovascular events by 40%.9
5. Annually reassess the ratio of benefit to risk
At every patient’s annual visit, weigh the reasons for hormone therapy against current knowledge of the benefit-risk ratio, which evolves along with the patient’s needs and status. When all the data from the WHI studies are available and reassessed, the benefit-risk ratio is likely to change again, further altering our recommendations. In my practice I explain the current data and remind patients that the most important—if not the only—reason for ET/EPT is to control menopausal symptoms.
To determine whether the menopausal symptoms have resolved or have become more tolerable, I invite the patient to discontinue the therapy for 4 to 8 weeks. If the symptoms recur and are intolerable, or if the patient concludes that the ratio of benefits to risks is positive, we resume therapy. If she feels that ratio is negative, we stop and consider alternatives.
Our role should be helping the patient arrive at her own conclusions based on the scientific data that we provide, as well as the symptoms she experiences.
Recommendations for initiating therapy
As has been stated, the only indications for EPT in menopausal women are vasomotor symptoms and associated quality-of-life issues. When the therapy is given for these reasons, women should be advised to take the lowest effective dose of the more physiologic preparations for as short a time as possible.
Again, the duration of therapy must be individualized and regularly assessed. This may require periodic interruption of the therapy to evaluate symptom recurrence and the patient’s tolerance of and response to safer alternatives.
Estrogen. I favor estradiol—either orally or transdermally—starting at daily doses of 0.5 mg or 0.035 mg, respectively. If symptoms are not adequately controlled and serum estradiol levels remain below 50 pcg/mL, I increase the daily dose to 0.75 mg or 0.075 mg, respectively. If necessary, I will increase it again to a maximum of 1 mg or 0.100 mg, respectively.
Asymptomatic women do not need—nor will they benefit from—ET/EPT.
Progestin. For the progestin component, I recommend 200 mg of oral micronized progesterone, to be taken at bedtime for 2 weeks of each or every other month to confer endometrial protection. If patients cannot tolerate micronized progesterone, I recommend norethindrone acetate in combination with estradiol, to be administered orally (Activella) or transdermally (CombiPatch).
Dr. Luciano reports that he receives research/grant support from Proctor & Gamble, Chitogenics, ML Labs, and TAP; is a consultant to Eli Lilly and Pharmacia; and serves on the speaker’s bureau of Eli Lilly and Pharmacia.
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
2. American College of Obstetricians and Gynecologists. Response to Women’s Health Initiative Study Results by the American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
3. Grodstein F, Manson JE, Golditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
4. Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstet Gynecol. 1994;84:787-793.
5. DeLeo V, La Marca A, Morgante G, Lanzetta D. Comparison of 2 HRT regimens with bimonthly and monthly progestin administration in postmenopause. Maturitas. 1999;31:171-177.
6. Ettinger B, Selby J, Citron JT, Vangessel A, Ettinger VM, Hendrickson MR. Cyclic hormone replacement using quarterly progestin. Obstet Gynecol. 1994;83:693-700.
7. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-645.
8. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from MORE trial. Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
9. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: Four-year results from the MORE randomized trial. JAMA. 2002;287:847-857.
- Give estrogen and estrogen-progestin therapy only for the relief of significant vasomotor symptoms, and halt the therapy in all asymptomatic women.
- Prescribe natural estrogens and progesterone whenever possible and measure serum levels to assess response and compliance.
- Initiate therapy at a dose of 0.3 mg for conjugated equine estrogen, 0.5 mg for oral estradiol, and 0.035 mg for transdermal estradiol and progressively increase, if necessary, to no more than twice these amounts.
- Give progesterone cyclically rather than continuously to reduce risk of cardiovascular disease, breast cancer, other adverse events.
- Reassess regimens annually in each patient.
I replied that most of my symptomatic postmenopausal patients continued taking estrogen therapy (ET) or EPT. Still, I understood the concern that prompted the question.
After the WHI findings became widely publicized, many women discontinued EPT—only to resume therapy 8 to 10 weeks later because of persistent vasomotor symptoms. A number of other postmenopausal women, however, were able to cope with the transient recurrence of menopausal symptoms. I was happy to encourage them to permanently discontinue ET/EPT, since the primary reason for starting the therapy (vasomotor symptoms) was no longer an issue and the secondary reason (long-term benefits originally attributed to therapy with estrogen and progestin) had been seriously challenged.
This article discusses the American College of Obstetricians and Gynecologists guidelines on ET/EPT,2 and includes 5 specific pointers on managing menopausal women at risk for osteoporosis, breast cancer, and cardiovascular disease.
The bottom line: The WHI has significantly affected clinical practice, but hormone use is not precluded in symptomatic post-menopausal women, whose distressing symptoms and quality of life are improved by EPT.
TABLE 1
Relative risk of selected adverse events in women taking estrogen-progestin therapy1
| EVENT | RELATIVE RISK | CONFIDENCE INTERVAL | STATISTICAL SIGNIFICANCE |
|---|---|---|---|
| Coronary heart disease | 1.29 | 1.02–1.63 | Yes |
| Stroke | 1.41 | 1.07–1.85 | Yes |
| Breast cancer | 1.26 | 1.00–1.59 | Yes |
| Pulmonary embolism | 2.13 | 1.39–3.25 | Yes |
| Endometrial cancer | 0.83 | 0.47–1.47 | No |
| Hip fracture | 0.66 | 0.45–0.98 | Yes |
| Colorectal cancer | 0.63 | 0.43–0.92 | Yes |
| Deep venous thrombosis | 2.07 | 1.49–2.87 | Yes |
| Vertebral fractures | 0.66 | 0.44–0.98 | Yes |
| Other osteoporotic fractures | 0.77 | 0.69–0.86 | Yes |
| Global index | 1.15 | 1.03–1.28 | Yes |
TABLE 2
Number of selected adverse events per 10,000 woman-years1*
| EVENT | PLACEBO (N = 8,102) | CEE/MPA (N=8,506) | DELTA |
|---|---|---|---|
| Coronary heart disease | 30 | 37 | 7 |
| Stroke | 21 | 29 | 9 |
| Breast cancer | 30 | 38 | 8 |
| Pulmonary embolism | 8 | 16 | 8 |
| Endometrial cancer | 6 | 5 | -1 |
| Hip fracture | 15 | 10 | -5 |
| Colorectal cancer | 16 | 10 | -6 |
| Deep venous thrombosis† | 3 | 26 | 16 |
| Vertebral fractures† | 15 | 9 | -6 |
| Other osteoporotic fractures† | 170 | 131 | -49 |
| Global index | 151 | 170 | 19 |
| CEE = conjugated equine estrogen | |||
| MPA = medroxyprogesterone acetate | |||
| *Absolute risk | |||
| †Not included in global index | |||
1. Individualize treatment
In the realm of estrogen therapy, 1 size does not fit all. Women tolerate and respond differently to various preparations and doses. I tend to use estradiol and micronized progesterone as much as possible because I believe they may be safer. With natural hormones it also is easier to measure serum levels; this helps me assess a patient’s response to and compliance with therapy.
I prefer the transdermal preparation consisting of estradiol/norethindrone acetate (CombiPatch) because it is more physiologic and yields constant serum levels throughout the 24-hour day. In addition, it avoids the first pass through the liver, which is associated with nonphysiologic alteration of hepatic enzymes and proteins—especially binding globulins for steroids, thyroid and sex hormones, and coagulation factors.
2. Use the lowest effective dose to achieve benefits and avoid side effects
This is a critical recommendation. Although this rule should apply to all therapies, it is especially important for ET/EPT, which can be associated with life-threatening side effects.
In a prospective, observational study conducted in healthy postmenopausal women, Grodstein et al3 explored primary prevention of cardiovascular disease. They found that the relative risk of stroke rose with each incremental increase in the dose of unopposed conjugated equine estrogen (CEE). The relative risk of stroke with CEE at a daily dose of 0.3 mg, 0.625 mg, and 1.25 mg was 0.54 (95% confidence interval [CI], 0.28–1.06), 1.35 (95% CI, 1.08–1.68), and 1.63 (95% CI, 1.18–2.26), respectively. Thus, the lowest effective dose at initiation would be 0.3 mg for CEE. Comparable doses for oral estradiol and transdermal estradiol are 0.5 mg and 0.035 mg, respectively.
This dose may be progressively increased according to the patient’s response, but seldom should exceed double the starting dose. Similar guidelines should be followed for progestins.
3. Consider minimizing duration of progestin treatment
Prescribe ET for hysterectomized women and EPT for those with a uterus. This may seem like old advice, since all gynecologists know the importance of using EPT in women with a uterus. But the optimal formulation and administration of EPT remain elusive. It is now clear that not all progestins have the same physiologic effects—especially on cardiovascular health (see). For this reason, the type, sequence, dose, and duration of progestin administration should be carefully reevaluated.
Daily progestin is not physiologic. When it first became evident that unopposed estrogen causes endometrial cancer in women with a uterus, progestins were administered for 7, 10, or 12 days each month, usually in the form of medroxyprogesterone acetate (MPA), mimicking the cyclic production of progesterone by the premenopausal ovary. Although this regimen was effective in protecting against endometrial cancer, the cyclic administration of EPT induced undesirable uterine bleeding, which caused many women to stop the therapy. To avoid cyclic bleeding and improve compliance, continuous combined EPT preparations were introduced, which involved daily exposure to progestin—a level that is clearly not physiologic.
Because it appears from the WHI study that progestins may be mostly responsible for the increased risks of cardiovascular disease, stroke, and breast cancer—since the estrogen-alone arm of the trial continues—it may be prudent to minimize the duration of progestin treatment in postmenopausal women. Several studies have demonstrated that the cyclic administration of progestin for 2 weeks every 2 to 3 months may be as protective against endometrial cancer as monthly administration.4-6 Moreover, if they receive the lowest effective dose of daily estrogen (as outlined) and bi- or tri-monthly cyclic progesterone, most women will experience little or no bleeding.
Prior to the Women’s Health Initiative (WHI), estrogen therapy (ET) and estrogen-progestin therapy (EPT) were widely assumed to protect against cardiovascular disease. This assumption had been substantiated by epidemiologic studies, as well as experimental and clinical trials, which consistently reported that ET/EPT had beneficial effects on the lipid profile. Also noted were direct effects on vessel walls, evident in decreasing arteriosclerosis and increased vasodilatation.1 Were all these studies erroneous? Or is there an explanation for both pre- and post-WHI findings?
Observational studies are somewhat notorious for selection and compliance biases. Women who take hormones generally are healthier, better educated, and wealthier than nonusers; they also have fewer coronary risk factors. Thus, the favorable outcomes observed with ET and EPT may have simply reflected these women’s greater health at baseline. But I don’t think so.
Although these confounding factors may have exaggerated the benefits of ET/EPT, it is unlikely that they masked the increased risk observed with the WHI and other recently published prospective, randomized studies, such as the Heart and Estrogen/progestin Replacement Study (HERS)2 and the Women’s Estrogen for Stroke Trial (WEST).3 Moreover, the other benefits (osteoporosis and colon cancer protection) and risks (thromboembolic events and breast cancer) previously reported in observational studies have been appropriately confirmed by prospective, randomized studies. Only the cardiovascular benefits have not. Instead, they have been completely negated. Why?
There is no clear answer, but 1 explanation may be that the significant improvement in cardiovascular health care for women over the past 2 decades has obscured the cardiovascular benefits of EPT manifested 15 years ago in epidemiologic studies.
Indeed, 20 and 30 years ago, cardiovascular disease was considered a disease of men; very few women were evaluated or treated for hypertension or hyperlipidemia, 2 major cardiovascular disease risk factors. In women, ET/EPT may have been therapeutic, either indirectly by improving the lipid profile or directly by decreasing plaque formation and inducing vasodilatation through regulation of nitric oxide, prostaglandin synthetase, and membrane ionic permeability.1
During the past 15 years, women’s health care has changed greatly. Women who have hypertension or hyperlipidemia—especially those who participate in clinical trials2-4—are commonly treated with potent lipid-lowering agents and antihypertensive medications. These agents’ protective effects against cardiovascular disease would obscure any potential vascular benefits of ET/EPT. Consequently, only the adverse vascular effects of ET/EPT would be noted, since its potential benefits would have been usurped by the stronger cardiotropic statins and antihypertensive drugs.
Consider this corollary: Had the WHI patients at risk for fracture been treated with bisphosphonates, I doubt very much that the protective effects of ET/EPT on bone would have been noted. This hypothesis is supported by the recent Estrogen in the Prevention of Atherosclerosis Trial.5 In this prospective study, 222 post-menopausal women with low-density lipoprotein cholesterol levels in excess of 130 mg/dL were randomized to receive either 17-ß estradiol or placebo and were angiographically monitored for progression of coronary atherosclerosis.
The results revealed that the women randomized to 17-ß estradiol experienced significantly less progression of atherosclerosis than the placebo group. However, in those study patients who were also taking lipid-lowering therapy (statins), 17-ß estradiol did not confer additional benefits on coronary atherosclerosis.
It appears, then, that ET/EPT served its purpose for cardiovascular protection when there was a need for it—prior to the widespread use of cardioprotective agents. Now, with vastly improved women’s health care, ET/EPT for cardiovascular protection is neither needed nor safe.
Role of progestin. The fact that the estrogenonly arm of the WHI has not been interrupted supports the hypothesis that the progestin in the EPT preparation is mostly responsible for the increased risk of breast cancer and atherosclerosis, including coronary heart disease and stroke.
Moreover, medroxyprogesterone acetate (MPA)—the progestin used in the WHI—may be key to the cardiovascular risks6 observed in the trial. A different progestin, such as micronized progesterone or norethindrone acetate, may not confer similar risks.7 Indeed, the Postmenopausal Estrogen/Progestin Interventions study found that the addition of MPA to conjugated equine estrogen in postmenopausal women negated some of the beneficial effects of conjugated equine estrogen on the lipid profile, whereas the addition of micronized progesterone did not.8
Moreover, animal studies have found that MPA6—but not norethindrone acetate9 or micronized progesterone—negates the beneficial effects of estrogen on coronary plaque formation. Thus, it appears that different progestins have variable effects on atherosclerosis and that, among the various clinically available progestins, MPA may be particularly deleterious to cardiovascular health. Unfortunately, the results of these animal studies may not apply to humans. Still, they should serve as hypotheses for future prospective, randomized clinical trials.
Whether the WHI findings can be generalized to other hormone preparations is unclear. At present, the burden of proof for greater safety and efficacy lies with the sponsors of the other products.
REFERENCES
1. Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615-624.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
3. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
4. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
5. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. Ann Intern Med. 2001;135:939-953.
6. Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Artherioscler Thromb Vasc Biol. 1997;17:217-221.
7. Riis BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187:1101-1116.
8. Alexanderson P, Haarbo J, Sandholdt I, Shalmi M, Lawaetz H, Christiansen C. Norethindrone acetate enhances the antiatherogenic effect of 17-beta-estradiol: a secondary prevention study of aortic atherosclerosis in ovariectomized cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1998;18:902-907.
9. The Writing group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199-208.
4. Stop long-term, fixed-dose EPT in asymptomatic women
Also eliminate its use for cardiovascular protection. Asymptomatic women do not need—nor will they benefit from—ET/EPT, so there is no reason to use it. EPT’s potential benefit in terms of bone loss and fractures can be achieved with other agents that are FDA-approved for the prevention of osteoporosis but carry less risk of breast cancer, cardiovascular disease, or thromboembolic events.
During an interim analysis of the Women’s Health Initiative (WHI) estrogen-progestin arm, the data safety monitoring board determined that estrogen and progestin therapy (EPT) had a greater potential for harm than for benefit. So on July 9, 2002, after an average of 5.2 years of observation, that arm was halted.1
The other arm of the trial continued to examine the use of unopposed estrogen in hysterectomized women. The fact that it continues suggests that its global index is still not definitively unfavorable.
At the time the EPT arm was halted, its participants had an increased incidence of 26% for breast cancer, 41% for stroke, 29% for coronary heart disease events, and 110% for thromboembolic events, compared with the placebo group. On the benefit side, they experienced a 37% reduction in colorectal cancer and a 34% reduction in hip fractures. These reductions were not sufficient to offset the increased risk (TABLES 1 and 2). There was no difference in mortality rates between the EPT and placebo groups.
Absolute versus relative risk. Because relative risks are confusing and often misunderstood, the results were also reported in absolute values, expressed in the number of additional events for treated woman-years, which is more meaningful to individual women. Thus, among 10,000 women taking EPT for a year, there will be 8 more cases of invasive breast cancer, 8 more strokes, 8 more pulmonary embolic events, and 7 more myocardial infarctions, but 6 fewer cases of colorectal cancer and 5 fewer hip fractures.
The absolute risk for an individual woman remains quite small (less than 0.1% per year). When this risk was calculated for all events over the 5.2 years of the trial, approximately 100 more women in the hormone group experienced an adverse event than in the placebo group. If this figure is extrapolated to the population at large and to a longer treatment duration, the use of EPT could account for tens of thousands of additional adverse events.
Study excluded women with hot flashes. The WHI did not evaluate the relief of vasomotor symptoms. In fact, women with severe hot flashes were excluded from the study. This means that the major benefits and most common indication for EPT were not considered in the risk-benefit ratio. Had they been included, that ratio might have yielded different results.
Value of the data. Nevertheless, the WHI data do provide important information, which allows us to discuss the benefits and risks of EPT more precisely and more objectively, especially for asymptomatic postmenopausal women.
REFERENCE
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
- The bisphosphonates—alendronate and risedronate—are safe, effective, convenient (weekly dosing), and not associated with life-threatening side effects.
- Raloxifene, the selective estrogen receptor modulator, is another good alternative for prevention and treatment of osteoporosis in patients not at risk for thromboembolic events. Unlike ET/EPT, raloxifene is not associated with an increased risk of either breast cancer or cardiovascular events. In fact, data from the Multiple Outcomes of Raloxifene Evaluation study suggest that, besides protecting against bone loss and vertebral fractures,7 raloxifene may reduce the risk of breast cancer8 by as much as 75%.9 Nor was raloxifene found to increase the risk or incidence of cardiovascular disease.8 In fact, in a group of 1,035 postmenopausal women at high risk for cardiovascular disease, raloxifene reduced the incidence of all cardiovascular events by 40%.9
5. Annually reassess the ratio of benefit to risk
At every patient’s annual visit, weigh the reasons for hormone therapy against current knowledge of the benefit-risk ratio, which evolves along with the patient’s needs and status. When all the data from the WHI studies are available and reassessed, the benefit-risk ratio is likely to change again, further altering our recommendations. In my practice I explain the current data and remind patients that the most important—if not the only—reason for ET/EPT is to control menopausal symptoms.
To determine whether the menopausal symptoms have resolved or have become more tolerable, I invite the patient to discontinue the therapy for 4 to 8 weeks. If the symptoms recur and are intolerable, or if the patient concludes that the ratio of benefits to risks is positive, we resume therapy. If she feels that ratio is negative, we stop and consider alternatives.
Our role should be helping the patient arrive at her own conclusions based on the scientific data that we provide, as well as the symptoms she experiences.
Recommendations for initiating therapy
As has been stated, the only indications for EPT in menopausal women are vasomotor symptoms and associated quality-of-life issues. When the therapy is given for these reasons, women should be advised to take the lowest effective dose of the more physiologic preparations for as short a time as possible.
Again, the duration of therapy must be individualized and regularly assessed. This may require periodic interruption of the therapy to evaluate symptom recurrence and the patient’s tolerance of and response to safer alternatives.
Estrogen. I favor estradiol—either orally or transdermally—starting at daily doses of 0.5 mg or 0.035 mg, respectively. If symptoms are not adequately controlled and serum estradiol levels remain below 50 pcg/mL, I increase the daily dose to 0.75 mg or 0.075 mg, respectively. If necessary, I will increase it again to a maximum of 1 mg or 0.100 mg, respectively.
Asymptomatic women do not need—nor will they benefit from—ET/EPT.
Progestin. For the progestin component, I recommend 200 mg of oral micronized progesterone, to be taken at bedtime for 2 weeks of each or every other month to confer endometrial protection. If patients cannot tolerate micronized progesterone, I recommend norethindrone acetate in combination with estradiol, to be administered orally (Activella) or transdermally (CombiPatch).
Dr. Luciano reports that he receives research/grant support from Proctor & Gamble, Chitogenics, ML Labs, and TAP; is a consultant to Eli Lilly and Pharmacia; and serves on the speaker’s bureau of Eli Lilly and Pharmacia.
- Give estrogen and estrogen-progestin therapy only for the relief of significant vasomotor symptoms, and halt the therapy in all asymptomatic women.
- Prescribe natural estrogens and progesterone whenever possible and measure serum levels to assess response and compliance.
- Initiate therapy at a dose of 0.3 mg for conjugated equine estrogen, 0.5 mg for oral estradiol, and 0.035 mg for transdermal estradiol and progressively increase, if necessary, to no more than twice these amounts.
- Give progesterone cyclically rather than continuously to reduce risk of cardiovascular disease, breast cancer, other adverse events.
- Reassess regimens annually in each patient.
I replied that most of my symptomatic postmenopausal patients continued taking estrogen therapy (ET) or EPT. Still, I understood the concern that prompted the question.
After the WHI findings became widely publicized, many women discontinued EPT—only to resume therapy 8 to 10 weeks later because of persistent vasomotor symptoms. A number of other postmenopausal women, however, were able to cope with the transient recurrence of menopausal symptoms. I was happy to encourage them to permanently discontinue ET/EPT, since the primary reason for starting the therapy (vasomotor symptoms) was no longer an issue and the secondary reason (long-term benefits originally attributed to therapy with estrogen and progestin) had been seriously challenged.
This article discusses the American College of Obstetricians and Gynecologists guidelines on ET/EPT,2 and includes 5 specific pointers on managing menopausal women at risk for osteoporosis, breast cancer, and cardiovascular disease.
The bottom line: The WHI has significantly affected clinical practice, but hormone use is not precluded in symptomatic post-menopausal women, whose distressing symptoms and quality of life are improved by EPT.
TABLE 1
Relative risk of selected adverse events in women taking estrogen-progestin therapy1
| EVENT | RELATIVE RISK | CONFIDENCE INTERVAL | STATISTICAL SIGNIFICANCE |
|---|---|---|---|
| Coronary heart disease | 1.29 | 1.02–1.63 | Yes |
| Stroke | 1.41 | 1.07–1.85 | Yes |
| Breast cancer | 1.26 | 1.00–1.59 | Yes |
| Pulmonary embolism | 2.13 | 1.39–3.25 | Yes |
| Endometrial cancer | 0.83 | 0.47–1.47 | No |
| Hip fracture | 0.66 | 0.45–0.98 | Yes |
| Colorectal cancer | 0.63 | 0.43–0.92 | Yes |
| Deep venous thrombosis | 2.07 | 1.49–2.87 | Yes |
| Vertebral fractures | 0.66 | 0.44–0.98 | Yes |
| Other osteoporotic fractures | 0.77 | 0.69–0.86 | Yes |
| Global index | 1.15 | 1.03–1.28 | Yes |
TABLE 2
Number of selected adverse events per 10,000 woman-years1*
| EVENT | PLACEBO (N = 8,102) | CEE/MPA (N=8,506) | DELTA |
|---|---|---|---|
| Coronary heart disease | 30 | 37 | 7 |
| Stroke | 21 | 29 | 9 |
| Breast cancer | 30 | 38 | 8 |
| Pulmonary embolism | 8 | 16 | 8 |
| Endometrial cancer | 6 | 5 | -1 |
| Hip fracture | 15 | 10 | -5 |
| Colorectal cancer | 16 | 10 | -6 |
| Deep venous thrombosis† | 3 | 26 | 16 |
| Vertebral fractures† | 15 | 9 | -6 |
| Other osteoporotic fractures† | 170 | 131 | -49 |
| Global index | 151 | 170 | 19 |
| CEE = conjugated equine estrogen | |||
| MPA = medroxyprogesterone acetate | |||
| *Absolute risk | |||
| †Not included in global index | |||
1. Individualize treatment
In the realm of estrogen therapy, 1 size does not fit all. Women tolerate and respond differently to various preparations and doses. I tend to use estradiol and micronized progesterone as much as possible because I believe they may be safer. With natural hormones it also is easier to measure serum levels; this helps me assess a patient’s response to and compliance with therapy.
I prefer the transdermal preparation consisting of estradiol/norethindrone acetate (CombiPatch) because it is more physiologic and yields constant serum levels throughout the 24-hour day. In addition, it avoids the first pass through the liver, which is associated with nonphysiologic alteration of hepatic enzymes and proteins—especially binding globulins for steroids, thyroid and sex hormones, and coagulation factors.
2. Use the lowest effective dose to achieve benefits and avoid side effects
This is a critical recommendation. Although this rule should apply to all therapies, it is especially important for ET/EPT, which can be associated with life-threatening side effects.
In a prospective, observational study conducted in healthy postmenopausal women, Grodstein et al3 explored primary prevention of cardiovascular disease. They found that the relative risk of stroke rose with each incremental increase in the dose of unopposed conjugated equine estrogen (CEE). The relative risk of stroke with CEE at a daily dose of 0.3 mg, 0.625 mg, and 1.25 mg was 0.54 (95% confidence interval [CI], 0.28–1.06), 1.35 (95% CI, 1.08–1.68), and 1.63 (95% CI, 1.18–2.26), respectively. Thus, the lowest effective dose at initiation would be 0.3 mg for CEE. Comparable doses for oral estradiol and transdermal estradiol are 0.5 mg and 0.035 mg, respectively.
This dose may be progressively increased according to the patient’s response, but seldom should exceed double the starting dose. Similar guidelines should be followed for progestins.
3. Consider minimizing duration of progestin treatment
Prescribe ET for hysterectomized women and EPT for those with a uterus. This may seem like old advice, since all gynecologists know the importance of using EPT in women with a uterus. But the optimal formulation and administration of EPT remain elusive. It is now clear that not all progestins have the same physiologic effects—especially on cardiovascular health (see). For this reason, the type, sequence, dose, and duration of progestin administration should be carefully reevaluated.
Daily progestin is not physiologic. When it first became evident that unopposed estrogen causes endometrial cancer in women with a uterus, progestins were administered for 7, 10, or 12 days each month, usually in the form of medroxyprogesterone acetate (MPA), mimicking the cyclic production of progesterone by the premenopausal ovary. Although this regimen was effective in protecting against endometrial cancer, the cyclic administration of EPT induced undesirable uterine bleeding, which caused many women to stop the therapy. To avoid cyclic bleeding and improve compliance, continuous combined EPT preparations were introduced, which involved daily exposure to progestin—a level that is clearly not physiologic.
Because it appears from the WHI study that progestins may be mostly responsible for the increased risks of cardiovascular disease, stroke, and breast cancer—since the estrogen-alone arm of the trial continues—it may be prudent to minimize the duration of progestin treatment in postmenopausal women. Several studies have demonstrated that the cyclic administration of progestin for 2 weeks every 2 to 3 months may be as protective against endometrial cancer as monthly administration.4-6 Moreover, if they receive the lowest effective dose of daily estrogen (as outlined) and bi- or tri-monthly cyclic progesterone, most women will experience little or no bleeding.
Prior to the Women’s Health Initiative (WHI), estrogen therapy (ET) and estrogen-progestin therapy (EPT) were widely assumed to protect against cardiovascular disease. This assumption had been substantiated by epidemiologic studies, as well as experimental and clinical trials, which consistently reported that ET/EPT had beneficial effects on the lipid profile. Also noted were direct effects on vessel walls, evident in decreasing arteriosclerosis and increased vasodilatation.1 Were all these studies erroneous? Or is there an explanation for both pre- and post-WHI findings?
Observational studies are somewhat notorious for selection and compliance biases. Women who take hormones generally are healthier, better educated, and wealthier than nonusers; they also have fewer coronary risk factors. Thus, the favorable outcomes observed with ET and EPT may have simply reflected these women’s greater health at baseline. But I don’t think so.
Although these confounding factors may have exaggerated the benefits of ET/EPT, it is unlikely that they masked the increased risk observed with the WHI and other recently published prospective, randomized studies, such as the Heart and Estrogen/progestin Replacement Study (HERS)2 and the Women’s Estrogen for Stroke Trial (WEST).3 Moreover, the other benefits (osteoporosis and colon cancer protection) and risks (thromboembolic events and breast cancer) previously reported in observational studies have been appropriately confirmed by prospective, randomized studies. Only the cardiovascular benefits have not. Instead, they have been completely negated. Why?
There is no clear answer, but 1 explanation may be that the significant improvement in cardiovascular health care for women over the past 2 decades has obscured the cardiovascular benefits of EPT manifested 15 years ago in epidemiologic studies.
Indeed, 20 and 30 years ago, cardiovascular disease was considered a disease of men; very few women were evaluated or treated for hypertension or hyperlipidemia, 2 major cardiovascular disease risk factors. In women, ET/EPT may have been therapeutic, either indirectly by improving the lipid profile or directly by decreasing plaque formation and inducing vasodilatation through regulation of nitric oxide, prostaglandin synthetase, and membrane ionic permeability.1
During the past 15 years, women’s health care has changed greatly. Women who have hypertension or hyperlipidemia—especially those who participate in clinical trials2-4—are commonly treated with potent lipid-lowering agents and antihypertensive medications. These agents’ protective effects against cardiovascular disease would obscure any potential vascular benefits of ET/EPT. Consequently, only the adverse vascular effects of ET/EPT would be noted, since its potential benefits would have been usurped by the stronger cardiotropic statins and antihypertensive drugs.
Consider this corollary: Had the WHI patients at risk for fracture been treated with bisphosphonates, I doubt very much that the protective effects of ET/EPT on bone would have been noted. This hypothesis is supported by the recent Estrogen in the Prevention of Atherosclerosis Trial.5 In this prospective study, 222 post-menopausal women with low-density lipoprotein cholesterol levels in excess of 130 mg/dL were randomized to receive either 17-ß estradiol or placebo and were angiographically monitored for progression of coronary atherosclerosis.
The results revealed that the women randomized to 17-ß estradiol experienced significantly less progression of atherosclerosis than the placebo group. However, in those study patients who were also taking lipid-lowering therapy (statins), 17-ß estradiol did not confer additional benefits on coronary atherosclerosis.
It appears, then, that ET/EPT served its purpose for cardiovascular protection when there was a need for it—prior to the widespread use of cardioprotective agents. Now, with vastly improved women’s health care, ET/EPT for cardiovascular protection is neither needed nor safe.
Role of progestin. The fact that the estrogenonly arm of the WHI has not been interrupted supports the hypothesis that the progestin in the EPT preparation is mostly responsible for the increased risk of breast cancer and atherosclerosis, including coronary heart disease and stroke.
Moreover, medroxyprogesterone acetate (MPA)—the progestin used in the WHI—may be key to the cardiovascular risks6 observed in the trial. A different progestin, such as micronized progesterone or norethindrone acetate, may not confer similar risks.7 Indeed, the Postmenopausal Estrogen/Progestin Interventions study found that the addition of MPA to conjugated equine estrogen in postmenopausal women negated some of the beneficial effects of conjugated equine estrogen on the lipid profile, whereas the addition of micronized progesterone did not.8
Moreover, animal studies have found that MPA6—but not norethindrone acetate9 or micronized progesterone—negates the beneficial effects of estrogen on coronary plaque formation. Thus, it appears that different progestins have variable effects on atherosclerosis and that, among the various clinically available progestins, MPA may be particularly deleterious to cardiovascular health. Unfortunately, the results of these animal studies may not apply to humans. Still, they should serve as hypotheses for future prospective, randomized clinical trials.
Whether the WHI findings can be generalized to other hormone preparations is unclear. At present, the burden of proof for greater safety and efficacy lies with the sponsors of the other products.
REFERENCES
1. Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615-624.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
3. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
4. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
5. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. Ann Intern Med. 2001;135:939-953.
6. Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Artherioscler Thromb Vasc Biol. 1997;17:217-221.
7. Riis BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187:1101-1116.
8. Alexanderson P, Haarbo J, Sandholdt I, Shalmi M, Lawaetz H, Christiansen C. Norethindrone acetate enhances the antiatherogenic effect of 17-beta-estradiol: a secondary prevention study of aortic atherosclerosis in ovariectomized cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1998;18:902-907.
9. The Writing group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199-208.
4. Stop long-term, fixed-dose EPT in asymptomatic women
Also eliminate its use for cardiovascular protection. Asymptomatic women do not need—nor will they benefit from—ET/EPT, so there is no reason to use it. EPT’s potential benefit in terms of bone loss and fractures can be achieved with other agents that are FDA-approved for the prevention of osteoporosis but carry less risk of breast cancer, cardiovascular disease, or thromboembolic events.
During an interim analysis of the Women’s Health Initiative (WHI) estrogen-progestin arm, the data safety monitoring board determined that estrogen and progestin therapy (EPT) had a greater potential for harm than for benefit. So on July 9, 2002, after an average of 5.2 years of observation, that arm was halted.1
The other arm of the trial continued to examine the use of unopposed estrogen in hysterectomized women. The fact that it continues suggests that its global index is still not definitively unfavorable.
At the time the EPT arm was halted, its participants had an increased incidence of 26% for breast cancer, 41% for stroke, 29% for coronary heart disease events, and 110% for thromboembolic events, compared with the placebo group. On the benefit side, they experienced a 37% reduction in colorectal cancer and a 34% reduction in hip fractures. These reductions were not sufficient to offset the increased risk (TABLES 1 and 2). There was no difference in mortality rates between the EPT and placebo groups.
Absolute versus relative risk. Because relative risks are confusing and often misunderstood, the results were also reported in absolute values, expressed in the number of additional events for treated woman-years, which is more meaningful to individual women. Thus, among 10,000 women taking EPT for a year, there will be 8 more cases of invasive breast cancer, 8 more strokes, 8 more pulmonary embolic events, and 7 more myocardial infarctions, but 6 fewer cases of colorectal cancer and 5 fewer hip fractures.
The absolute risk for an individual woman remains quite small (less than 0.1% per year). When this risk was calculated for all events over the 5.2 years of the trial, approximately 100 more women in the hormone group experienced an adverse event than in the placebo group. If this figure is extrapolated to the population at large and to a longer treatment duration, the use of EPT could account for tens of thousands of additional adverse events.
Study excluded women with hot flashes. The WHI did not evaluate the relief of vasomotor symptoms. In fact, women with severe hot flashes were excluded from the study. This means that the major benefits and most common indication for EPT were not considered in the risk-benefit ratio. Had they been included, that ratio might have yielded different results.
Value of the data. Nevertheless, the WHI data do provide important information, which allows us to discuss the benefits and risks of EPT more precisely and more objectively, especially for asymptomatic postmenopausal women.
REFERENCE
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
- The bisphosphonates—alendronate and risedronate—are safe, effective, convenient (weekly dosing), and not associated with life-threatening side effects.
- Raloxifene, the selective estrogen receptor modulator, is another good alternative for prevention and treatment of osteoporosis in patients not at risk for thromboembolic events. Unlike ET/EPT, raloxifene is not associated with an increased risk of either breast cancer or cardiovascular events. In fact, data from the Multiple Outcomes of Raloxifene Evaluation study suggest that, besides protecting against bone loss and vertebral fractures,7 raloxifene may reduce the risk of breast cancer8 by as much as 75%.9 Nor was raloxifene found to increase the risk or incidence of cardiovascular disease.8 In fact, in a group of 1,035 postmenopausal women at high risk for cardiovascular disease, raloxifene reduced the incidence of all cardiovascular events by 40%.9
5. Annually reassess the ratio of benefit to risk
At every patient’s annual visit, weigh the reasons for hormone therapy against current knowledge of the benefit-risk ratio, which evolves along with the patient’s needs and status. When all the data from the WHI studies are available and reassessed, the benefit-risk ratio is likely to change again, further altering our recommendations. In my practice I explain the current data and remind patients that the most important—if not the only—reason for ET/EPT is to control menopausal symptoms.
To determine whether the menopausal symptoms have resolved or have become more tolerable, I invite the patient to discontinue the therapy for 4 to 8 weeks. If the symptoms recur and are intolerable, or if the patient concludes that the ratio of benefits to risks is positive, we resume therapy. If she feels that ratio is negative, we stop and consider alternatives.
Our role should be helping the patient arrive at her own conclusions based on the scientific data that we provide, as well as the symptoms she experiences.
Recommendations for initiating therapy
As has been stated, the only indications for EPT in menopausal women are vasomotor symptoms and associated quality-of-life issues. When the therapy is given for these reasons, women should be advised to take the lowest effective dose of the more physiologic preparations for as short a time as possible.
Again, the duration of therapy must be individualized and regularly assessed. This may require periodic interruption of the therapy to evaluate symptom recurrence and the patient’s tolerance of and response to safer alternatives.
Estrogen. I favor estradiol—either orally or transdermally—starting at daily doses of 0.5 mg or 0.035 mg, respectively. If symptoms are not adequately controlled and serum estradiol levels remain below 50 pcg/mL, I increase the daily dose to 0.75 mg or 0.075 mg, respectively. If necessary, I will increase it again to a maximum of 1 mg or 0.100 mg, respectively.
Asymptomatic women do not need—nor will they benefit from—ET/EPT.
Progestin. For the progestin component, I recommend 200 mg of oral micronized progesterone, to be taken at bedtime for 2 weeks of each or every other month to confer endometrial protection. If patients cannot tolerate micronized progesterone, I recommend norethindrone acetate in combination with estradiol, to be administered orally (Activella) or transdermally (CombiPatch).
Dr. Luciano reports that he receives research/grant support from Proctor & Gamble, Chitogenics, ML Labs, and TAP; is a consultant to Eli Lilly and Pharmacia; and serves on the speaker’s bureau of Eli Lilly and Pharmacia.
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
2. American College of Obstetricians and Gynecologists. Response to Women’s Health Initiative Study Results by the American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
3. Grodstein F, Manson JE, Golditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
4. Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstet Gynecol. 1994;84:787-793.
5. DeLeo V, La Marca A, Morgante G, Lanzetta D. Comparison of 2 HRT regimens with bimonthly and monthly progestin administration in postmenopause. Maturitas. 1999;31:171-177.
6. Ettinger B, Selby J, Citron JT, Vangessel A, Ettinger VM, Hendrickson MR. Cyclic hormone replacement using quarterly progestin. Obstet Gynecol. 1994;83:693-700.
7. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-645.
8. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from MORE trial. Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
9. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: Four-year results from the MORE randomized trial. JAMA. 2002;287:847-857.
1. Writing group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:321-323.
2. American College of Obstetricians and Gynecologists. Response to Women’s Health Initiative Study Results by the American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2002.
3. Grodstein F, Manson JE, Golditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
4. Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstet Gynecol. 1994;84:787-793.
5. DeLeo V, La Marca A, Morgante G, Lanzetta D. Comparison of 2 HRT regimens with bimonthly and monthly progestin administration in postmenopause. Maturitas. 1999;31:171-177.
6. Ettinger B, Selby J, Citron JT, Vangessel A, Ettinger VM, Hendrickson MR. Cyclic hormone replacement using quarterly progestin. Obstet Gynecol. 1994;83:693-700.
7. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-645.
8. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from MORE trial. Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65(2):125-134.
9. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: Four-year results from the MORE randomized trial. JAMA. 2002;287:847-857.
SERMs: Protection without worry?
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
- Among ER-positive breast cancer patients treated with tamoxifen for 5 years, the annual recurrence rate is cut in half and the annual death rate is reduced by 28%.
- Tamoxifen has been shown to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 and older, respectively.
- Raloxifene mimics the effects of estrogen on the skeleton and lipids, but acts as a complete estrogen antagonist in the breast and uterus.
- Recent data indicate that 4 years of raloxifene therapy reduces the risk of all ER-positive breast cancers by 72% compared with placebo.
- The ideal candidate for raloxifene is a postmenopausal woman with osteopenia or osteoporosis, no increased risk of thromboembolism, and few or no vasomotor symptoms.
Although it was initially considered a female sex hormone, estrogen is now recognized as a systemic substance that affects every organ system and appears to be important for both men’s and women’s health.1-4 At puberty, elevated circulating estrogen levels transform girls into young women and shape their feminine fig-ures. In boys, estrogen is important in the closure of the epiphyseal plates to arrest postpubertal growth and support bone health throughout life. It also may have beneficial actions on the male cardiovascular system.2
As a systemic hormone, estrogen is important in women for the health of the skeleton, the heart, the integuments, and the brain.1,3 Women who suffer the loss of estrogen through surgery, illness, or early menopause often are advised to consider estrogen therapy to protect these organs from premature failure.
Unfortunately, estrogen replacement therapy (ERT) is a double-edged sword, with significant benefits in some organs and significant risks in others. ERT protects against vasomotor symptoms, urogenital atrophy, osteoporosis, cardiovascular disease, and perhaps Alzheimer’s disease.1,3 Unfortunately, it also increases the risk of venous thromboembolic events, recurrent myocardial infarction and cardiac death5 (in diseased hearts), and cancers of the uterus and breast.4
Of the many diseases that impact the lives of American women, none is more feared than breast cancer, the most common cancer in females and the second leading cause of female cancer mortality.6 The fear of this disease causes many women to decline or discontinue ERT, a decision that can profoundly affect their long-term health.7 Hence, the controversy regarding ERT centers on weighing the risk-benefit balance, i.e., trying to select patients who are more likely to benefit from the hormone and less likely to suffer from its adverse effects.
Physicians—particularly gynecologists who devote their care exclusively to women’s health—have longed for the ideal estrogen that will impart all of the benefits without the risks. This ideal estrogen would relieve women of vasomotor and urogenital symptoms and prevent the dire consequences of osteoporosis, accelerated atherosclerosis, neurogenic deficit, and collagen loss from the skin, without increasing the risk of cancer and thromboembolic phenomena. Some experts believe this ideal may be found in the new class of compounds referred to as selective estrogen receptor modulators (SERMs). Although several SERMs with desirable estrogenic properties are available, none is ideal. Nevertheless, this new class of estrogenic substances—with multiple beneficial effects and fewer risks—represents an important pharma-cotherapeutic advance in the care of postmenopausal women.
Tamoxifen
Tamoxifen citrate (Nolvadex; AstraZeneca, Wilmington, Del) has wide clinical applications in the treatment and prevention of breast cancer. In 1980, tamoxifen was approved by the FDA for postmenopausal women with node-positive breast cancer and for premenopausal women with estrogen-receptor (ER) positive advanced breast cancer. In 1990, the FDA extended the approval of tamoxifen to include pre- and postmenopausal women with node-negative, ER-positive breast cancer.
In patients with node-positive breast cancer, the 10-year-survival rate improves from 50% in control subjects to 61% in patients treated with tamoxifen for 5 years. Similar improvements in survival have been reported with tamoxifen therapy in node-negative breast cancer patients. After 5 years of tamoxifen therapy and a median follow-up of 10 years, the reduction of breast cancer recurrence and death with tamoxifen treatment compared with placebo are 47% and 26%, respectively.8
For women with ER-positive tumors (about 70% of all breast cancers), tamoxifen therapy of at least 1 year’s duration results in statistically significant recurrence and survival benefits. These results increase with the duration of treatment up to 5 years. Among ER-positive women treated with tamoxifen for 5 years—the optimal length of therapy—the annual recurrence rate is cut in half and the annual death rate is reduced by 28% (Figure 1).9 Treatment beyond 5 years does not accrue additional benefits and is associated with increased adverse events.10 Tamoxifen has little effect on animal tumors and ER-negative tumors in cell cultures and confers little or no benefit to women with ER-negative tumors.9
In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.
Figure 2 shows the mean changes in BMD and the corresponding reduction in vertebral fractures for the therapeutic agents currently approved for the prevention and treatment of osteoporosis. Despite significant differences in BMD increases, the reductions in vertebral fracture rates are quite comparable. In the MORE trial, there was no difference in the incidence of nontraumatic fractures at sites other than the spine, possibly because of the small number of nonvertebral fractures that occurred during the study period, which yielded insufficient power to detect a difference. A subset of patients in the MORE study is being followed for a longer time to further evaluate the incidence of nonvertebral fractures.
Effects on cardiovascular health. Raloxifene’s effects on total and low-density lipoprotein (LDL) cholesterol are similar to those of estrogen, with decreases of 11% and 6%, respectively. Unlike estrogen, which increases the circulating levels of both high-density lipoprotein (HDL) cholesterol and triglycerides, raloxifene causes an insignificant increase in HDL and a slight decrease in triglyceride levels. Serum levels of lipoprotein(a) are reduced much more with ERT (16.3%) than raloxifene therapy (4.1%), whereas fibrinogen levels are reduced more with raloxifene (12.2%) than ERT (2.8%).15
The effects of raloxifene on 2 additional independent risk factors for cardiovascular disease were recently reported. Elevated levels of C-reactive protein, a circulating marker of inflammation, are associated with a significantly greater risk of myocardial infarction. Also, elevated levels of homocysteine predict a greater risk of coronary artery disease. While ERT significantly increases circulating levels of C-reactive protein (by as much as 84%), raloxifene lacks a significant effect. Like estrogen, raloxifene significantly lowers homocysteine levels by 6% to 8%.16
The changes in serum levels of the several markers of cardiovascular health are summarized for both ERT and raloxifene in Table 1. Overall, raloxifene therapy is associated with favorable changes in the serum levels of several of these markers, suggesting that the SERM may substantially reduce the risk of heart disease in postmenopausal women. However, conclusive proof would require a clinical trial with cardiovascular events as the definitive endpoints. Such an investigation is currently being carried out in the Raloxifene Use for the Heart (RUTH) trial. The results should indicate whether these favorable biochemical effects are indeed associated with a reduction in the incidence of cardiovascular disease.
Effects on the endometrium. Unlike tamoxifen and toremifene (Fareston; Schering-Plough, Kenilworth, NJ), which stimulate endometrial proliferation and increase the risk of endometrial cancer, raloxifene has no stimulatory effects on either the endometrium or the myometrium.17,18 In postmenopausal women receiving raloxifene, the incidence of vaginal bleeding is the same as in women on placebo. In the MORE trial, raloxifene did not increase the risk of endometrial cancer, endometrial hyperplasia, or uterine bleeding over a follow-up period of 40 months.20 Several other studies have evaluated the endometrial response to raloxifene—compared with placebo or ERT—for periods ranging from 12 to 24 months. These studies have consistently reported that raloxifene has no stimulatory effects on the uterus of postmenopausal women.17,18,21 Thus, it seems clear that postmenopausal patients on raloxifene need not worry about an increased risk of polyps, endometrial proliferation, hyperplasia, or cancer. Their endometrial thickness and uterine volume will not increase, nor will they experience a greater incidence of vaginal bleeding than untreated women.5,17,18,20
In postmenopausal women, the administration of raloxifene does not require the addition of a progestin or routine endometrial monitoring by biopsy or ultrasonography. Any bleeding during raloxifene therapy should therefore be promptly evaluated, since it is unlikely to be related to the SERM.
Effects on the breast. In contrast to ERT, raloxifene does not increase the frequency of breast pain or tenderness in postmenopausal women. In addition, recent data suggest that raloxifene may reduce the risk of breast cancer.
Like tamoxifen, raloxifene inhibits the growth of mammary tumors in rodents and the growth of the human MCF-7 breast cancer cell line in vitro.9,22 The latest follow-up data from the MORE trial indicate that 4 years of raloxifene therapy reduces the risk of all breast cancers by 72% compared with placebo. At the 4-year mark of the MORE trial, a total of 79 breast cancer cases occurred—4.7 cases per 1,000 patient-years in the placebo group compared with 1.3 cases per 1,000 patient-years in the raloxifene group. The protective effect was essentially restricted to ER-positive cancers. As in previous observations, women with the highest serum estrogen levels had the highest BMD and the highest incidence of breast cancer. These same women experienced the greatest reduction in breast cancer risk with raloxifene therapy.
Because raloxifene has not yet been studied directly as a prophylactic, it cannot be recommended for the prevention of breast cancer. Several other issues regarding raloxifene and breast cancer prevention also need to be addressed. For example, the efficacy rates of raloxifene in women at increased risk for breast cancer and in younger postmenopausal women are unknown. We also lack data regarding the optimal duration of raloxifene therapy for maximal, sustained protection against breast cancer.
These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.
The ideal candidate for raloxifene appears to be a postmenopausal woman with osteopenia or osteoporosis who has no increased risk of thromboembolism and few or no vasomotor symptoms. Raloxifene would be especially suitable for such a candidate if she has experienced any bleeding on ERT or fears an increased risk of breast cancer. This would include women who have been on ERT for 5 years or more, since the use of ERT for more than 5 years significantly increases the risk of breast cancer.4
In my practice, the women who are happiest with raloxifene tend to be older (4 or more years past menopause), concerned about osteoporosis and cardiovascular symptoms, and very glad to be free of vaginal bleeding while on the drug. Since the 2 most common reasons for discontinuing ERT—vaginal bleeding and the fear of breast cancer—are non-issues when raloxifene is given, compliance is likely to be high. The few cases of vaginal dryness that occur usually can be treated with lubricants, small doses of vaginal estrogen tablets, or the vaginal estrogen ring.
TABLE 3
Treatment options for osteoporosis: benefits and side effects
| Agent | Benefits | Side effects |
|---|---|---|
| ERT | Effective, safe, multidimensional.* Eases vasomotor and urogenital symptoms, improves mood, protects cardiovascular health | Bleeding, blood clots, breast cancer |
| Raloxifene | Effective, safe, multidimensional. Improves lipid profile. No bleeding or breast cancer risk | Blood clots. No relief of hot flushes or vaginal dryness |
| Alendronate, risedronate | Effective, safe, multidimensional | Difficult to take. Esophageal and gastric irritation, bleeding |
| Calcitonin | Effective, safe, unidimensional. Analgesic effects in patients with compression fractures | Insignificant |
| *The term “multidimensional” suggests that the agent affects more than 1 organ system. | ||
Conclusion
Thanks to a dramatic increase in life expectancy, American women can expect to live 30 or more years beyond the average age of menopause. With aging, the risk of health problems increases progressively. Several of these problems are specifically related to or augmented by estrogen deficiency. Despite several health benefits of ERT, its use remains very low (less than 30% in postmenopausal women) because of side effects and concerns about safety, bleeding, and breast cancer.7
SERMs—particularly raloxifene—provide many of estrogen’s positive effects on bone metabolism and lipids and other markers of cardiovascular disease without increasing the risk of bleeding or breast cancer. In fact, recent data suggest that raloxifene not only fails to increase the risk of breast cancer but actually may be protective against the disease.
SERMs do not necessarily replace older therapies such as ERT, but they do enhance our ability to modify and improve therapies, individualize regimens, and boost compliance. This is especially important with long-term preventive therapy, which requires a high level of acceptance and commitment by both patient and physician. Because of their multiple beneficial effects and favorable long-term risk-benefit profile, SERMs represent an important therapeutic advance in the field of women’s health.
Dr. Luciano reports that he serves on the speakers’ bureaus for Eli Lilly and Co., Wyeth Labs, and Pharmacia, and that he receives research grants from Pharmacia.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.
1. Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in menopausal women. Ann Intern Med. 1992;117(12):1016-1037.
2. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogen in men. J Clin Endocrinol Metab. 1999;84:3411-3415.
3. Barrett-Connors E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annu Rev Public Health. 1998;19:55-72.
4. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059.
5. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
6. Cancer Facts and Figures—1997. Atlanta: American Cancer Society; 1997.
7. Berman RS, et al. Risk factors associated with women’s compliance with estrogen replacement therapy. J Women’s Health. 1997;6:219-226.
8. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
9. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
10. Fisher B, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542.
11. Fisher B, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
12. Baker VL, Leitman D, Jaffe RB. Selective estrogen receptor modulators in reproductive medicine and biology. Obstet Gynecol Survey. 2000;55(7 Suppl 2):S21-S47.
13. Early Breast Cancer Collaborative Trial Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451-1467.
14. Ettinger B, Black D, Mitlak B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644.
15. Walsh B, Kuller L, Wild R, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451.
16. Walsh B, Paul S, Wild R, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218.
17. Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
18. Cohen FJ, Watts S, Shah A, et al. Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104-110.
19. Delmas P, Bjarnason N, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
20. Cummings S, Eckert S, Krueger K, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA. 1999;281:2189-2197.
21. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977-980.
22. Bryant H, Glasebrook A, Yang N, et al. A pharmacologic review of raloxifene. J Bone Miner Metab. 1995;13:75-78.
23. Davies G, et al. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93:558-565.
24. Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216.
25. Evista [package insert]. Indianapolis, Ind: Eli Lilly and Company; 1997.