User login
Getting PrEP to the patients who need it
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5
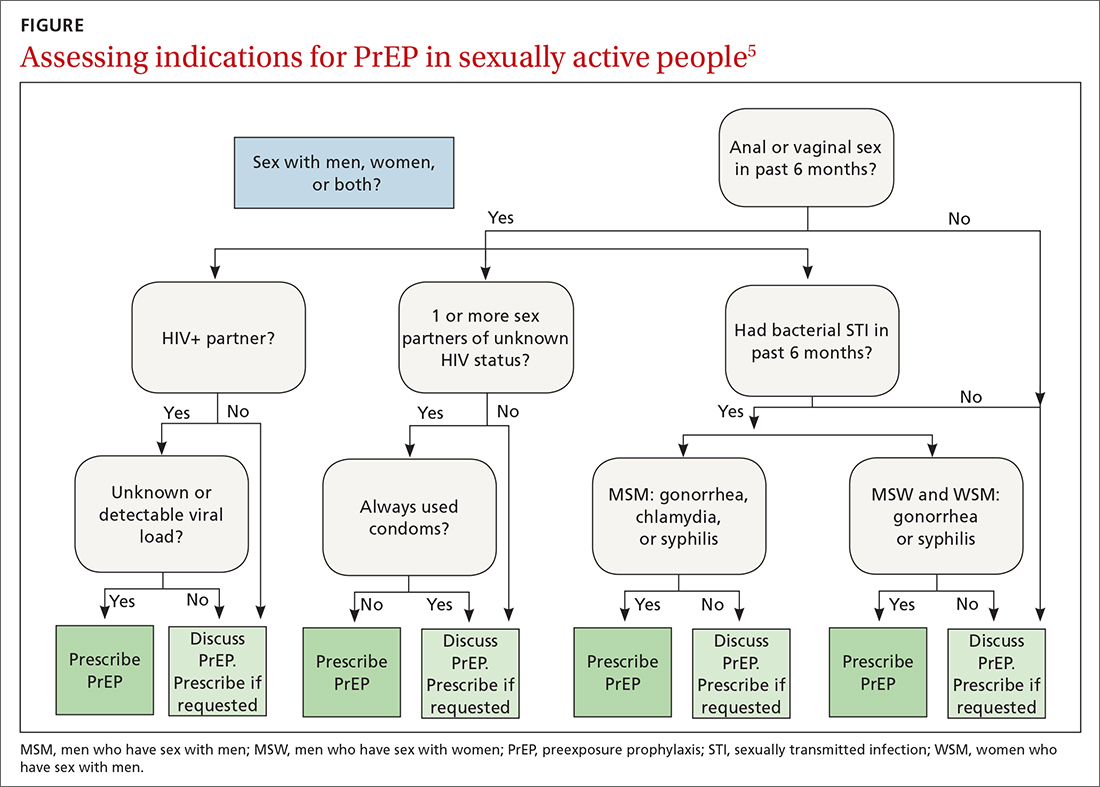
PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.
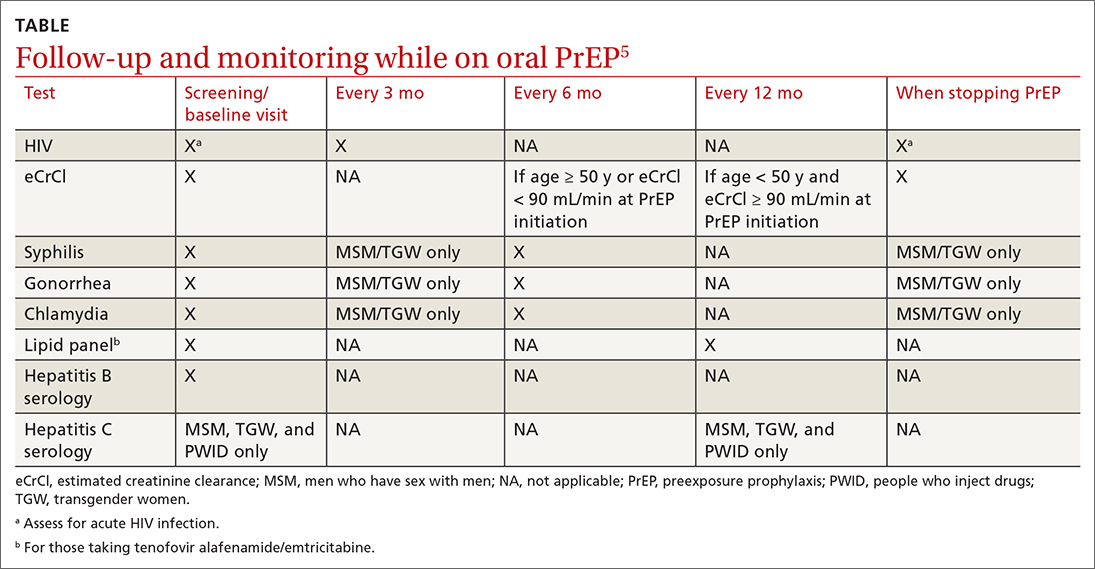
Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5

PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.

Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
More than 1.2 million Americans are living with HIV, and more than 30,000 new cases are diagnosed each year. While total incidence has declined since 2016, HIV remains a nationwide epidemic.1
Medications that prevent HIV acquisition, termed preexposure prophylaxis (PrEP), are an important tool to initiate in the primary care setting to reduce HIV transmission. However, while there are an estimated 1.2 million people eligible for PrEP, only 36% have received PrEP prescriptions.2 Several barriers that have impeded its widespread adoption include a lack of clinician knowledge and clinical resources for testing, high medication costs, and stigma around sexual health and intravenous (IV) drug use.
The value of PrEP
PrEP is chemoprophylaxis against the acquisition of HIV infection through the administration of an oral or injectable medication to people at risk for HIV. This practice began in the early 2000s, with the first oral regimen approved in 2012, and since has become an important tool in preventing HIV transmission.
When taken as prescribed, PrEP medications reduce the risk for acquiring HIV through sex by approximately 99% and can reduce the risk for acquiring HIV from injection drug use by approximately 74%.3 The US Preventive Services Task Force issued a Grade “A” recommendation to offer PrEP to people at high risk for HIV acquisition in June 2019 and reaffirmed it in a 2023 update.4
PrEP is notably distinct from postexposure prophylaxis (PEP), which is the administration of medication to prevent HIV infection after a possible exposure.
The available regimens
Regimens for PrEP include oral tablets or intramuscular (IM) injections.5 There are 3 PrEP regimens approved by the US Food and Drug Administration (FDA): tenofovir disoproxil fumarate/emtricitabine (Truvada), tenofovir alafenamide/emtricitabine (Descovy), and cabotegravir (Apretude).
Truvada is once-daily oral PrEP that was approved in 2012 and is now available in a generic formulation. Notable adverse effects of Truvada include a small negative impact on renal function and small reductions in bone mineral density; these have been noted in individual trials, but in meta-analyses such differences were not found to be statistically significant.6-8 The most common adverse effects of Truvada, experienced by up to 6% of patients, are gastrointestinal symptoms, fatigue, headache/dizziness, depression, and insomnia; most symptoms resolve within weeks.
Continue to: Descovy
Descovy is daily oral PrEP that was approved in 2019. Descovy is associated with increases in LDL and triglycerides but has less impact on renal and bone health.9 The most common adverse effect of Descovy, experienced by about 5% of patients, is diarrhea, followed by nausea.
Apretude was approved in 2021 and is a 600-mg IM injection given monthly for 2 months, then every 2 months (± 7 days). The advantages of Apretude are frequency and discreteness of dosing and the ability to use in patients with estimated creatinine clearance (eCrCl) > 15 mL/min. The most common adverse effects of Apretude are injection-site reactions, which occur in 30% to 80% of patients but are rarely significant enough to lead to discontinuation (< 2% of patients discontinue use due to injection-site reactions).10
Who should take PrEP?
The latest Centers for Disease Control and Prevention (CDC) guidelines recommend that all sexually active adults receive information about PrEP.5 Indications for PrEP are broad and summarized in the FIGURE.5

PrEP is indicated in patients who report sexual or injection drug use behaviors that place them at substantial ongoing risk for HIV exposure. Specific indications include patients with sexual partner(s) with unknown HIV status with whom they have inconsistent or no condom use, a history of bacterial sexually transmitted infection (STI) in the past 6 months, an HIV-positive sexual partner, or the sharing of injection drug equipment.
Hepatitis B infection is not a contraindication for PrEP use, but knowledge of infection status is essential. All current oral medications used for PrEP have activity against hepatitis B. Incomplete adherence to or abrupt discontinuation of oral PrEP could precipitate a hepatitis B flare. Hepatitis B surface antigen should be tested at the time of PrEP initiation, although PrEP can begin while testing is in process.
Continue to: How to use PrEP
How to use PrEP
At PrEP initiation, acute or chronic HIV infection must be excluded with a documented negative HIV antigen/antibody test within 1 week of prescribing PrEP.5 The CDC guidelines provide an updated HIV testing algorithm (www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf, p 30-31, Figures 4a and 4b), which considers whether patients have received PrEP recently.
Patients with recent high-risk exposures or symptoms of acute HIV at the time of desired PrEP initiation should have an HIV-1 viral load checked with negative results before PrEP is prescribed. Additional criteria for PrEP include weight > 35 kg; screening for hepatitis B virus infection; screening for drug interactions; and drug-specific eCrCl cutoffs of > 60 mL/min for Truvada, > 30 mL/min for Descovy, and > 15 mL/min for Apretude.5
Studies regarding time to medication effectiveness are limited. Pharmacokinetic studies of Truvada demonstrate sufficient drug concentrations should be present in peripheral blood mononuclear cells and rectal tissue within 7 days of initiation of oral dosing and around 20 days in vaginal tissue.
Of note, while expedited partner therapy is used as a harm-reduction strategy to treat the sexual partners of patients diagnosed with certain STIs, PrEP is not recommended to be used in this way.
Ongoing monitoring with PrEP. Once oral PrEP is started, STI risk assessment and HIV testing via 4th generation antibody/antigen test should be completed at least every 3 months. PrEP oral prescription refills should be limited to 3 months. For patients receiving IM PrEP (Apretude), HIV testing via viral load and antibody/antigen testing should be done at the time of each injection (every 2 months).5
Continue to: With oral PrEP...
With oral PrEP, renal function should be checked every 6 months in patients older than 50 years or those with eCrCl < 90 mL/min at initiation. For patients younger than 50 years with no baseline renal dysfunction, the latest guidelines now recommend monitoring every 12 months instead of 6 months.5
For patients on Descovy, a lipid panel is recommended at PrEP initiation and every 12 months. Testing for other STIs can be considered on this schedule, based on clinical assessment. The TABLE5 summarizes recommended monitoring for patients taking oral PrEP.

Recommended follow-up provides an opportunity to have frequent contact with a potentially high-risk population, and PrEP should be one part of a comprehensive HIV prevention and risk reduction plan. Many patients at high risk for HIV acquisition may benefit from frequent follow-up to address screening, referral, and treatment of substance use disorders, mental health conditions, and chronic medical conditions (including hepatitis C infection) and provide ongoing preventive health care.
Special uses of PrEP
Same-day PrEP. Starting PrEP on the day of the initial appointment may be appropriate based on patient risk factors and barriers to care, such as a high risk for contracting HIV before the subsequent appointment for a prescription of PrEP or an inability to return to the clinic in a timely fashion due to transportation or work constraints, or clinician availability. For these patients, assuming there is a low concern for acute or chronic HIV infection, PrEP can be initiated on the day of the initial visit.5
In these cases, point-of-care HIV and creatinine testing with same-day results should be completed. Antigen/antibody fingerstick testing or HIV-1 RNA test are preferred; oral fluid HIV testing should not be used for same-day PrEP due to its lower sensitivity for HIV detection. If same-day testing is unavailable, blood should be drawn at the visit so that HIV and creatinine testing can be completed as soon as possible.
Continue to: In addition to initial laboratory testing...
In addition to initial laboratory testing, clinics offering same-day PrEP should be able to provide: (1) assistance for patients to enroll in health insurance or a medication assistance program (eg, Ready, Set, PrEP) for those ineligible for insurance coverage, (2) rapid follow-up on all laboratory results with reliable patient contact information, and (3) follow-up appointments with clinicians able to prescribe and administer PrEP medications.
Off-label “on-demand” PrEP. An off-label treatment regimen for men who have sex with men (MSM) is termed “on-demand” PrEP or “2-1-1 PrEP” and is included in the CDC guidelines for consideration by clinicians.5 This alternative dosing schedule can be used for individuals who have sex less frequently and in a more planned fashion.
On-demand PrEP requires a patient to take 2 tablets of Truvada 2 to 24 hours before sex, followed by 1 tablet 24 hours and 1 tablet 48 hours after sexual activity. If a sexual act occurs at 48 hours, the patient should extend the daily dose for 48 additional hours, such that PrEP is always used daily for 48 hours after the last sex act.
This method has been studied with Truvada in MSM in Europe and Canada through the IPERGAY and PREVENIR trials and shown to have ≥ 86% efficacy in preventing HIV acquisition.11,12 The only US-based study showed lower efficacy; however, based on the currently available data, the International Antiviral Society-USA Panel has recommended it as an alternative regimen.13,14
PrEP via telehealth. Visits for PrEP initiation and continuation can be completed via telehealth.5 Patients then can complete necessary laboratory tests by going to a physical laboratory location or using mailed specimen kits in which they can self-collect urine, oral/rectal swabs, and fingerstick blood samples.
Continue to: PrEP use in specific populations
PrEP use in specific populations
Adolescents
Truvada, Descovy, and Apretude all are now approved for use in adolescents weighing ≥ 35 kg. Two important considerations when prescribing to this population are the effects of Truvada on bone health and the unique barriers to access.
In studies of adolescent MSM using Truvada for PrEP, bone mineral density declined, especially among those ages 15 to 19 years.15 As such, the clinical impact of decreased bone mineral density should be weighed against the risk for HIV acquisition; however, bone mineral density monitoring is not recommended in the current guidelines. CDC guidelines suggest considering Descovy for male adolescents given its potential lower impact on bone mineral density.5
Confidentiality and legal issues exist when prescribing PrEP to minors. In terms of parental/guardian involvement, clinicians who are prescribing PrEP for patients younger than 18 years should consult the CDC website for guidance on local and state regulations that govern prescribing and confidentiality (www.cdc.gov/hiv/policies/law/states/minors.html).
Insurance billing statements may lead to inadvertent disclosure of a minor’s decision to take PrEP to their legal guardian.16 Generic Truvada costs less than $100 for a 3-month supply when using goodrx.com, which may offer an alternative to insurance for medication payment.
Peripartum patients
The increased risk for HIV acquisition in the peripartum period for female patients is well documented.17 Guidelines recommend offering PrEP with Truvada to female patients at risk for conception, currently pregnant, or breastfeeding when that patient’s partner has HIV and the partner’s viral load is unknown or detectable. Descovy is not recommended for pregnant or breastfeeding patients.5 Cabotegravir-containing regimens (Apretude) have not been approved by the FDA for pregnant or breastfeeding patients.5
Continue to: Data on the impact of...
Data on the impact of Truvada for PrEP on fetal health are still emerging. A large study in Kenya showed no significant differences in preterm birth, low birth weight, or early infant growth, and a randomized, noninferiority trial in South Africa showed no association between Truvada for PrEP and preterm birth or the birth of small-for-gestational-age infants.18,19 There are no definitive studies of breastfeeding infants exposed to Truvada, but data from previous trials of breastfeeding mothers who were taking the individual components that are combined in the Truvada pill indicated there is minimal medication exposure to the infant.5
PrEP studies in the peripartum period to date have been conducted exclusively among cisgender women, and data do not yet reflect the experiences of transgender men, genderqueer people, and nonbinary individuals in the peripartum period.5
Transgender people
Transgender women should be strongly considered candidates for PrEP as they are at an extremely high risk for HIV acquisition. The most recent National HIV Behavioral Surveillance survey found that approximately 42% of transgender women were living with HIV.20 The survey revealed stark racial and ethnic disparities among transgender women living with HIV: 62% identified as Black/African American, compared with 35% Hispanic/Latina and 17% White.20
Transgender women report high rates of sexual assault, unprotected receptive anal sex, commercial sex work, homelessness, mental health disorders, and substance use, putting them at increased risk for HIV acquisition.21 However, transgender women are less likely to have discussed PrEP with a clinician, are less likely to be on PrEP even when interested in starting, and have higher rates of medication nonadherence compared with cisgender MSM.21,22 PrEP has not been found to decrease levels of feminizing hormones; however, studies are mixed as to whether feminizing hormones decrease Truvada concentrations in rectal mucosa, so clinicians should emphasize the importance of daily medication adherence.23
Transgender men have not been included in any PrEP trials, so no specific recommendations are available.
Continue to: Disparities in PrEP access and use exist
Disparities in PrEP access and use exist
The lifetime risk for HIV acquisition is 9% among White MSM, 50% among Black MSM, and 20% among Hispanic MSM.24 Despite this large disparity in disease burden, Black and Hispanic individuals are less likely to be aware of PrEP, have discussed PrEP with a health care professional, or used PrEP compared with their White counterparts.25 As a result, in 2020, PrEP coverage for eligible White individuals was 61%, while coverage among eligible Black and Hispanic/Latino individuals was just 8% and 14%, respectively.26
Surveillance data comparing male and female PrEP coverage reveal further disparities between the sexes, with PrEP coverage for eligible female-at-birth patients estimated to be 9% compared with 25.8% for male-at-birth patients.26 The gap between the risk for HIV infection and the access to and uptake of PrEP coverage is most pronounced among Black women, for whom the rate of new HIV diagnosis is > 10 times higher than it is for White women, but who have some of the lowest awareness and utilization rates of all demographics.27
The rural population at risk. Disparities in HIV awareness and PrEP use also exist between rural and urban populations, as well as by health insurance status. Rural areas have been shown to lag behind urban areas in PrEP awareness and use. Two potential explanations for this disparity are differences in HIV- and drug use–associated stigma and health insurance status. Greater stigma against drug use and HIV in rural areas has been associated with lower rates of PrEP use.28
Individuals younger than 65 years in rural areas are less likely to have private health insurance and more likely to be uninsured compared with their urban counterparts, which may impact access to clinicians knowledgeable about PrEP.29 Notably, MSM who live in states that have expanded Medicaid have higher rates of PrEP use compared with MSM living in states that have not expanded Medicaid.30
Health insurers in the United States are required to cover PrEP medication, clinician visits, and associated blood work with no patient cost-sharing, although implementation barriers such as prior authorizations still exist.
Conclusion
Family physicians are well positioned to identify patients at risk for HIV infection, prescribe PrEP, organize comprehensive follow-up care, and partner with their health systems and local communities to reduce barriers to care. Those who can leverage existing relationships with local health departments, school-based health clinics, congregate housing programs, LGBTQIA+ advocacy groups, harm-reduction coalitions, and other community-based organizations to raise PrEP awareness play a critical role in preventing HIV transmission and reducing health care disparities in their communities.
CORRESPONDENCE
Andrew V.A. Foley, MD, MPH, Erie Family Health Centers, 2418 W Division Street, Chicago, IL 60622; andrewvafoley@gmail.com
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
1. CDC. Estimated HIV incidence and prevalence in the United States 2017–2021. HIV Surveill Supplemental Rep. 2023;28. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
2. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through March 2023; and preexposure prophylaxis (PrEP) data reported through December 2022. HIV Surveill Data Tables. 2023;4. Published June 2023. Accessed October 23, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/
3. CDC. Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. PrEP effectiveness. Updated June 2022. Accessed October 23, 2023. https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html
4. US Preventive Services Task Force. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. August 22, 2023. Accessed October 23, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
5. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
6. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246-254. doi: 10.1001/jamainternmed.2014.6786
7. Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017;64:317-325. doi: 10.1093/cid/ciw765
8. Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215-224.
9. Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239-254. doi: 10.1016/S0140-6736(20)31065-5
10. Liegeon G, Ghosn, J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention. HIV Med. 2022;24:653-663. doi: 10.1111/hiv.13451
11. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246. doi: 10.1056/NEJMoa1506273
12. Molina JM, Ghosn J, Assoumou L, et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9:e554-e562. doi: 10.1016/S2352-3018(22)00133-3
13. Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and nondaily preexposure prophylaxis for men who have sex with men based on sex and pill-taking patterns from the Human Immuno Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2020;71:249-255. doi: 10.1093/cid/ciz799
14. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651-1669. doi: 10.1001/jama.2020.17025
15. Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis. 2020;70:687-691. doi: 10.1093/cid/ciz486
16. Neilan AM, Salvant Valentine S, Knopf AS. Case 27-2021: a 16-year-old boy seeking human immunodeficiency virus prophylaxis. N Engl J Med. 2021;385:1034-1041. doi: 10.1056/NEJMcpc1909626
17. Thomson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis. 2018;218:16-25. doi: 10.1093/infdis/jiy113
18. Dettinger JC, Kinuthia J, Pintye J, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. 2019;22:e25378. doi: 10.1002/jia2.25378
19. Moodley D, Lombard C, Govender V, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. 2023;10:e154-e163. doi: 10.1016/S2352-3018(22)00369-1
20. CDC. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. April 2021. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-27.pdf
21. Wilson EC, Turner CM, Arayasirikul S, et al. Disparities in the PrEP continuum for trans women compared to MSM in San Francisco, California: results from population-based cross-sectional behavioural surveillance studies. J Int AIDS Soc. 2020;23:e25539. doi: 10.1002/jia2.25539
22. Poteat T, Wirtz A, Malik M, et al. A gap between willingness and uptake: findings from mixed methods research on HIV prevention among Black and Latina transgender women. J Acquir Immune Defic Syndr. 2019;82:131-140. doi: 10.1097/QAI.0000000000002112
23. Cottrell ML, Prince HM, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for human immunodeficiency virus preexposure prophylaxis. Clin Infect Dis. 2019;69:2201-2204. doi: 10.1093/cid/ciz290
24. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238-243. doi: 10.1016/j.annepidem.2017.02.003
25. Kanny D, Jeffries WL 4th, Chapin-Bardales J, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:801-806. doi: 10.15585/mmwr.mm6837a2
26. CDC. Core indicators for monitoring the Ending the HIV Epidemic initiative (early release): National HIV Surveillance System data reported through December 2020; and preexposure prophylaxis (PrEP) data reported through September 2020. HIV Surveill Data Tables. 2021;2. Accessed October 23, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance-data-tables/vol-2-no-2/cdc-hiv-surveillance-tables-vol-2-no-2.pdf
27. CDC. Diagnoses of HIV infection in the United States and dependent areas 2021: special focus profiles. Updated May 23, 2023. Accessed October 23, 2023. www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/content/special-focus-profiles.html
28. Walters SM, Frank D, Van Ham B, et al. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2021;26:1308-1320. doi: 10.1007/s10461-021-03488-2
29. Foutz J, Artiga S, Garfield R. The role of Medicaid in rural America [issue brief]. April 25, 2017. Accessed August 16, 2023. www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/
30. Baugher AR, Finlayson T, Lewis R, et al. Health care coverage and preexposure prophylaxis (PrEP) use among men who have sex with men living in 22 US cities with vs without Medicaid expansion, 2017. Am J Public Health. 2021;111:743-751. doi: 10.2105/AJPH.2020.306035
PRACTICE RECOMMENDATIONS
› Perform routine screening of patients for preexposure prophylaxis (PrEP) eligibility. B
› Prescribe oral or intramuscular PrEP for eligible patients after screening for HIV, other sexually transmitted infections, and hepatitis B, and establishing baseline renal function. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
