User login
2016 Update on contraception
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
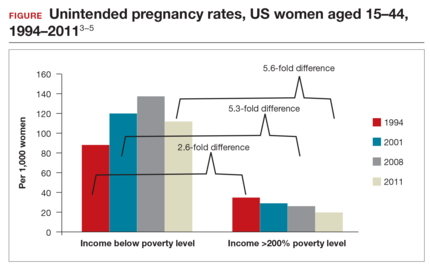
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5
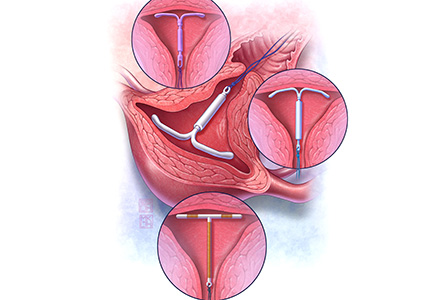
Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
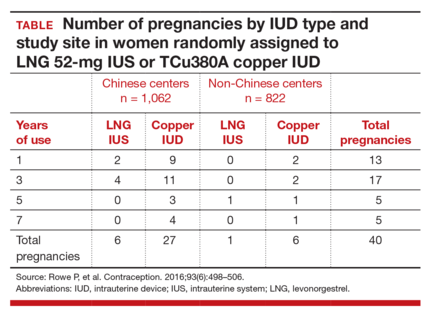
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7
Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
In this article
- Extended use of LNG IUS
- Oral LNG and LNG IUS combo for emergency contraception
- STI screening and same-day IUD placement