User login
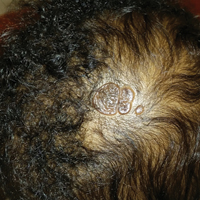
Yellow-Orange Hairless Plaque on the Scalp
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
The Diagnosis: Nevus Sebaceous
The patient presented with a typical solitary scalp lesion characteristic of nevus sebaceous (NS). The lesion was present at birth as a flat and smooth hairless plaque; however, over time it became more thickened and noticeable, which prompted the parents to seek medical advice.
Nevus sebaceous, also known as NS of Jadassohn, is a benign congenital hamartoma of the sebaceous gland that usually is present at birth and frequently involves the scalp and/or the face. The classic NS lesion is solitary and appears as a well-circumscribed, waxy, yellow-orange or tan, hairless plaque. Despite the presence of these lesions at birth, they may not be noted until early childhood or rarely until adulthood. Generally, the lesion tends to thicken and become more verrucous and velvety over time, particularly around the time of reaching puberty.1 Clinically, NS lesions vary in size from 1 cm to several centimeters. Lesions initially tend to grow proportionately with the child until puberty when they become notably thicker, greasier, and verrucous or nodular under hormonal influences. The yellow discoloration of the lesion is due to sebaceous gland secretion, and the characteristic color usually becomes less evident with age.
Nevus sebaceous occurs in approximately 0.3% of newborns and tends to be sporadic in nature; however, rare familial forms have been reported.2,3 Nevus sebaceous can present as multiple nevi that tend to be extensive and distributed along the Blaschko lines, and they usually are associated with neurologic, ocular, or skeletal defects. Involvement of the central nervous system frequently is associated with large sebaceous nevi located on the face or scalp. This association has been termed NS syndrome.4 Neurologic abnormalities associated with NS syndrome include seizures, mental retardation, and hemimegalencephaly.5 Ocular findings most communally associated with the syndrome are choristomas and colobomas.6-8
There are several benign and malignant epithelial neoplasms that may develop within sebaceous nevi. Benign tumors include trichoblastoma, syringocystadenoma papilliferum, trichilemmoma, sebaceoma, nodular hidradenoma, and hidrocystoma.1,8,9 Malignant neoplasms include basal cell carcinoma (BCC), apocrine carcinoma, sebaceous carcinoma, and squamous cell carcinoma. The lifetime risk of malignancy in NS is unknown. In an extensive literature review by Moody et al10 of 4923 cases of NS for the development of secondary benign and malignant neoplasms, 16% developed benign tumors while 8% developed malignant tumors such as BCC. However, subsequent studies suggested that the incidence of BCC may have been overestimated due to misinterpretation of trichoblastoma and may be less than 1%.11-13
Usually the diagnosis of NS is made clinically and rarely a biopsy for histopathologic confirmation may be needed when the diagnosis is uncertain. Typically, these histopathologic findings include immature hair follicles, hyperplastic immature sebaceous glands, dilated apocrine glands, and epidermal hyperplasia.9 For patients with suspected NS syndrome, additional neurologic and ophthalmologic evaluations should be performed including neuroimaging studies, skeletal radiography, and analysis of liver and renal function.14
The current standard of care in treating NS is full-thickness excision. However, the decision should be individualized based on patient age, extension and location of the lesion, concerns about the cosmetic appearance, and the risk for malignancy.
The 2 main reasons to excise NS include concern about malignancy and undesirable cosmetic appearance. Once a malignant lesion develops within NS, it generally is agreed that the tumor and the entire nevus should be removed; however, recommendations vary for excising NS prophylactically to decrease the risk for malignant growths. Because the risk for malignant transformation seems to be lower than previously thought, observation can be a reasonable choice for lesions that are not associated with cosmetic concern.12,13
Photodynamic therapy, CO2 laser resurfacing, and dermabrasion have been reported as alternative therapeutic approaches. However, there is a growing concern on how effective these treatment modalities are in completely removing the lesion and whether the risk for recurrence and potential for neoplasm development remains.1,9
This patient was healthy with normal development and growth and no signs of neurologic or ocular involvement. The parents were counseled about the risk for malignancy and the long-term cosmetic appearance of the lesion. They opted for surgical excision of the lesion at 18 months of age.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
- Eisen DB, Michael DJ. Sebaceous lesions and their associated syndromes: part I. J Am Acad Dermatol. 2009;61:549-560; quiz 561-562.
- Happle R, König A. Familial naevus sebaceus may be explained by paradominant transmission. Br J Dermatol. 1999;141:377.
- Hughes SM, Wilkerson AE, Winfield HL, et al. Familial nevus sebaceus in dizygotic male twins. J Am Acad Dermatol. 2006;54(2 suppl):S47-S48.
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26:221-230.
- Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol. 2002;43:20-23.
- Trivedi N, Nehete G. Complex limbal choristoma in linear nevus sebaceous syndrome managed with scleral grafting. Indian J Ophthalmol. 2016;64:692-694.
- Nema N, Singh K, Verma A. Complex limbal choristoma in nevus sebaceous syndrome [published online February 14, 2012]. Pediatr Dermatol. 2012;29:227-229.
- Park JM, Kim DS, Kim J, et al. Epibulbar complex choristoma and hemimegalencephaly in linear sebaceous naevus syndrome [published online July 2, 2009]. Clin Exp Dermatol. 2009;34:E686-E689.
- Simi CM, Rajalakshmi T, Correa M. Clinicopathologic analysis of 21 cases of nevus sebaceus: a retrospective study. Indian J Dermatol Venereol Leprol. 2008;74:625-627.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Rosen H, Schmidt B, Lam HP, et al. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26:676-681.
- Brandling-Bennett HA, Morel KD. Epidermal nevi. Pediatr Clin North Am. 2010;57:1177-1198.
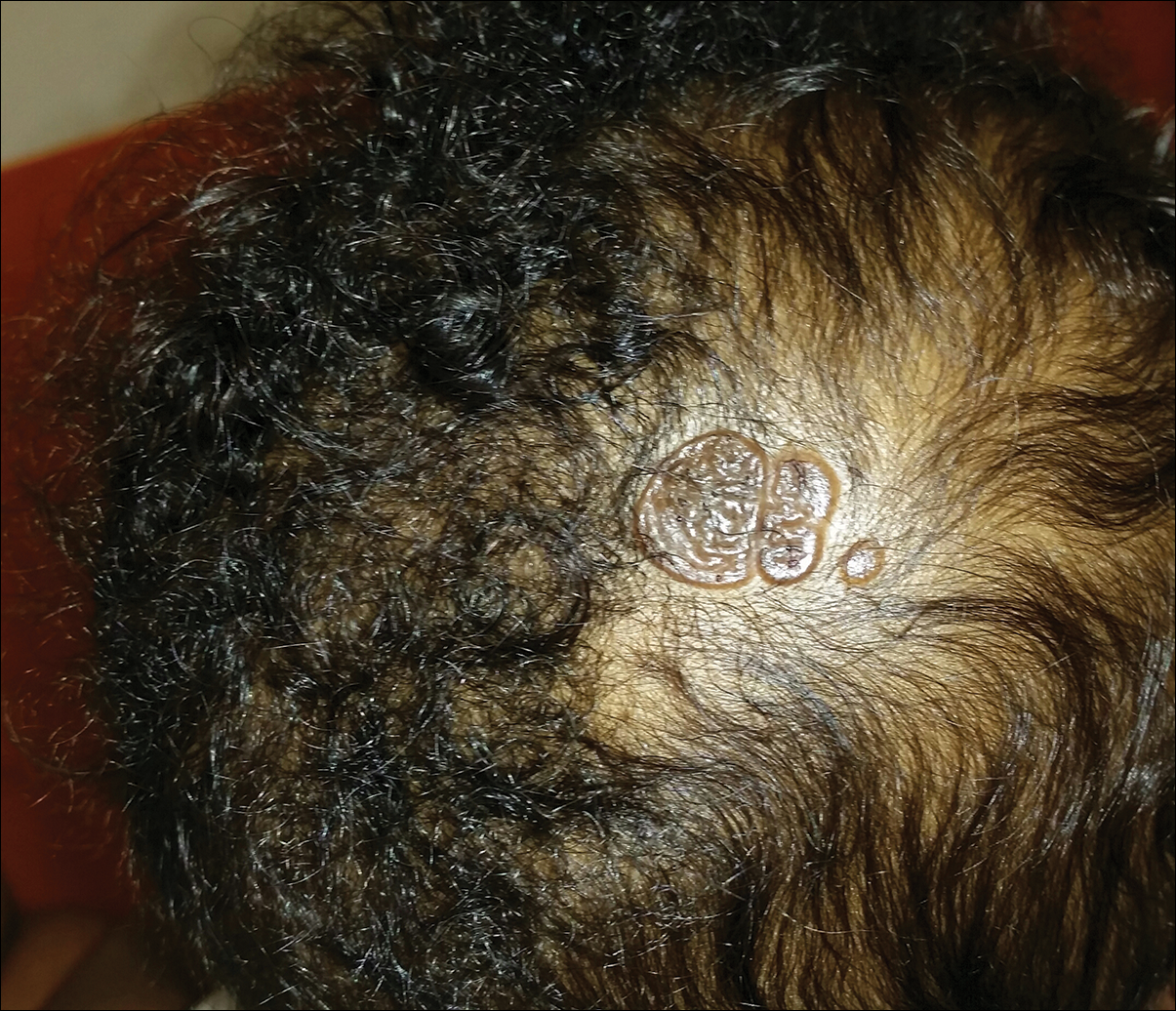
An otherwise healthy 13-month-old boy presented with a well-circumscribed, 3×4-cm, yellow-orange plaque with a verrucous velvety surface on the right side of the posterior scalp. The patient was born at 33 weeks' gestation and had an uneventful perinatal course with a normal head ultrasound at 4 days of age. The lesion had been present since birth and initially was comprised of waxy, yellow-orange, hairless plaques that became more thickened and noticeable over time. The mother recalled that the surface of the plaque initially was flat and smooth but gradually became bumpier and greasier in consistency in the months prior to presentation. The patient was otherwise asymptomatic.
Bilateral Onychodystrophy in a Boy With a History of Isolated Lichen Striatus
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
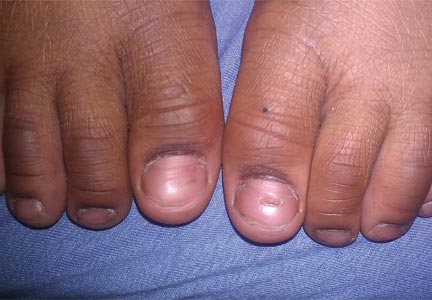
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
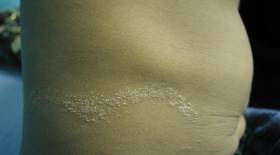
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
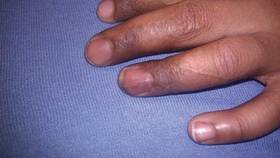
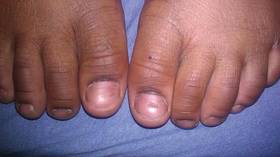
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
Practice Points
- Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology and diagnosis usually is made clinically.
- Nail involvement is uncommon in LS but also may be underreported. When present, nail changes may appear before, after, or concurrently with skin lesions.
- If a patient presents with a similar case of isolated onychodystrophy, the clinician should inquire about history of cutaneous LS and should consider the possibility of LS in the differential diagnosis.