User login
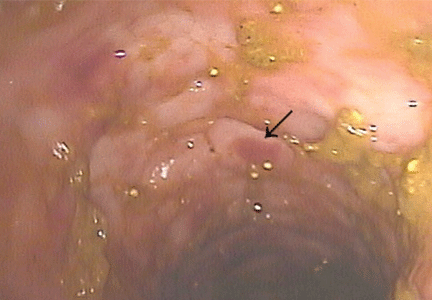
Cytomegalovirus colitis
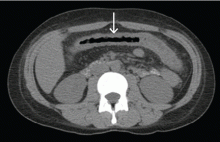
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
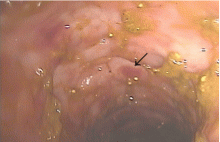
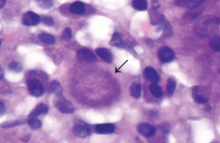
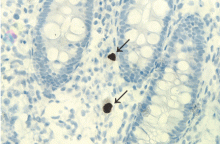
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.