User login
PPI Prophylaxis Prevents GI Bleed in Ventilated Patients
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
August 2024 – ICYMI
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
A Paradigm Shift in Evaluating and Investigating the Etiology of Bloating
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 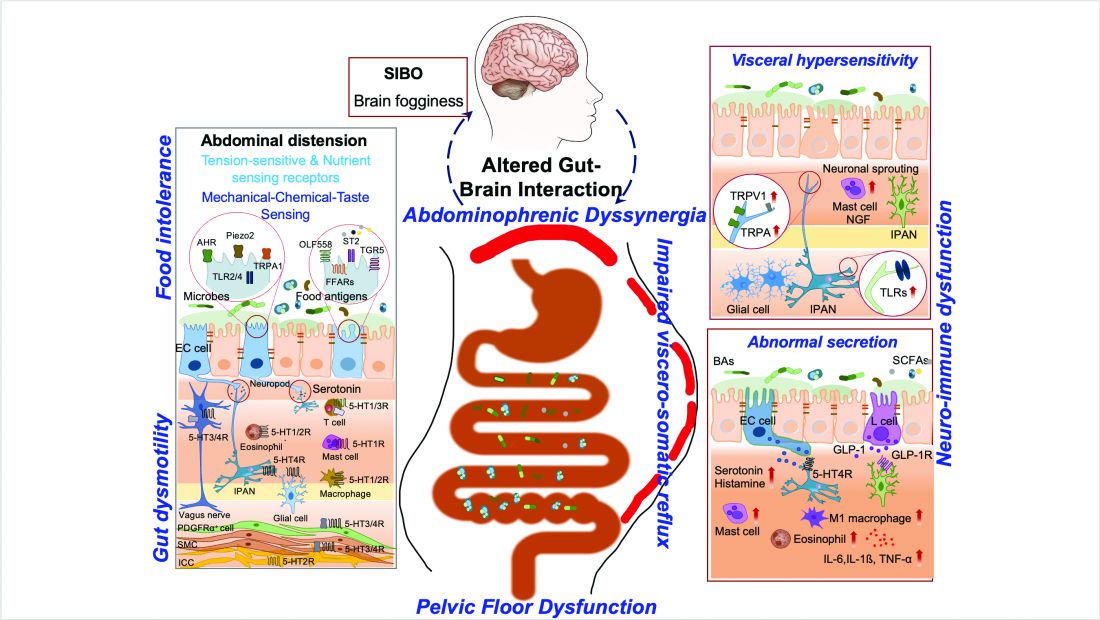
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
AGA Issues Guidance on Identifying, Treating Cyclic Vomiting Syndrome
, according to a new clinical practice update from the American Gastroenterological Association.
CVS affects up to 2% of U.S. adults and is more common in women, young adults, and those with a personal or family history of migraine headaches. However, most patients don’t receive a diagnosis or often experience years of delay in receiving effective treatment.
“A diagnosis is a powerful tool. Not only does it help patients make sense of debilitating symptoms, but it allows healthcare providers to create an effective treatment plan,” said author David J. Levinthal, MD, AGAF, director of the Neurogastroenterology and Motility Center at the University of Pittsburgh Medical Center.
“Our goal with this clinical practice update is to increase awareness of cyclic vomiting syndrome to reduce the diagnostic delay and increase patients’ access to treatment,” he said. “We hope to reach primary care, ER, and urgent care providers who are on the frontlines interacting with CVS patients seeking care, especially during an attack.”
The update was published online in Gastroenterology.
Understanding Cyclic Vomiting Syndrome
CVS is a chronic disorder of gut-brain interaction (DGBI), which is characterized by acute episodes of nausea and vomiting, separated by time without symptoms. Patients can usually identify a pattern of symptoms that show up during and between episodes.
CVS can vary, ranging from mild — with less than four episodes per year and lasting less than 2 days — to moderate-severe — with more than four episodes per year, lasting more than 2 days, and requiring at least one emergency department visit or hospitalization.
The disorder has four distinct phases — inter-episodic, prodromal, emetic, and recovery — that align with distinct treatment and management strategies. Between episodes, patients typically don’t experience repetitive vomiting but may experience symptoms such as mild nausea, indigestion, and occasional vomiting. Although CVS episodes can happen at any time, most tend to occur in the early morning.
For diagnosis, clinicians should consider CVS in adults presenting with episodic bouts of repetitive vomiting, following criteria established by the Rome Foundation. Rome IV criteria include acute-onset vomiting lasting less than 7 days, at least three discrete episodes in a year with two in the previous 6 months, and an absence of vomiting between episodes separated by at least 1 week of baseline health.
About 65% of patients with CVS experience prodromal symptoms, which last for about an hour before the onset of vomiting and may include panic, a sense of doom, and an inability to communicate effectively. During prodromal or emetic phases, patients have also reported fatigue, brain fog, restlessness, anxiety, headache, bowel urgency, abdominal pain, flushing, or shakiness.
As with migraines, CVS episodes may often be triggered by psychological and physiological factors, particularly stress. Episodes can stem from both negative stress, such as a death or relationship conflicts, as well as positive stress, such as birthdays and vacations. Other triggers include sleep deprivation, hormonal fluctuations linked to the menstrual cycle, travel, motion sickness, or acute infections.
Adult CVS is associated with several conditions, particularly mood disorders, including anxiety, depression, and panic disorder. Patients may also experience migraines, seizure disorders, or autonomic imbalances, such as postural orthostatic tachycardia syndrome, which may indicate pathophysiological mechanisms and routes for management.
The American Neurogastroenterology and Motility Society recommends testing to rule out similar or overlapping conditions, such as Addison’s disease, hypothyroidism, and hepatic porphyria. Diagnostic workup should include blood work, urinalysis, and one-time esophagogastroduodenoscopy or upper gastrointestinal imaging. Repeated imaging and gastric emptying scans should be avoided.
Providing Treatment and Prevention
For treatment, knowing the CVS phase is “essential,” the authors wrote. For instance, during the prodromal phase, abortive therapies can halt the transition to the emetic phase, and earlier intervention is associated with a higher probability of stopping an episode. The authors recommend intranasal sumatriptan, ondansetron, antihistamines, and sedatives.
During the emetic phase, supportive therapy can help terminate the episode. This may include continuing the abortive regimen and going to the emergency department for hydration and antiemetic medications. Patients may also find relief in a quiet, darker room in the emergency department, along with IV benzodiazepines, with the goal of inducing sedation.
During the recovery phase, patients should rest and focus on rehydration and nutrition to return to the well phase.
During the well or inter-episodic phase, patients can follow lifestyle measures to identify and avoid triggers, such as taking prophylactic medication (tricyclic antidepressants, anticonvulsants, and neurokinin-1 receptor antagonists such as aprepitant), reducing stress, and implementing a good sleep routine.
As part of patient education, clinicians can discuss the four phases and rehearse the actions to take to prevent or stop an episode.
“CVS has a significant impact on patients, families, and the healthcare system. The unpredictable and disruptive nature of episodes can result in reduced health-related quality of life, job loss precipitated by work absenteeism, and even divorce,” said Rosita Frazier, MD, a gastroenterologist at Mayo Clinic Arizona in Scottsdale who specializes in DGBI and CVS. Dr. Frazier, who wasn’t involved with the clinical practice update, has previously written about CVS diagnosis and management.
Patients with CVS often report negative interactions with physicians, particularly in the emergency department, where they may request specific treatments based on past experiences but are labeled as “drug seeking” and denied standard medical treatment, she said.
“Providing an individualized care plan for all patients could potentially address this problem and improve the physician-patient interaction,” she said. “Educational efforts to raise awareness among the medical community and increase both patient and provider engagement can optimize outcomes and are needed to address this critical problem.”
The authors received no specific funding for this update. Dr. Levinthal is a consultant for Takeda Pharmaceuticals and Mahana. Dr. Frazier reported no relevant financial disclosures.
, according to a new clinical practice update from the American Gastroenterological Association.
CVS affects up to 2% of U.S. adults and is more common in women, young adults, and those with a personal or family history of migraine headaches. However, most patients don’t receive a diagnosis or often experience years of delay in receiving effective treatment.
“A diagnosis is a powerful tool. Not only does it help patients make sense of debilitating symptoms, but it allows healthcare providers to create an effective treatment plan,” said author David J. Levinthal, MD, AGAF, director of the Neurogastroenterology and Motility Center at the University of Pittsburgh Medical Center.
“Our goal with this clinical practice update is to increase awareness of cyclic vomiting syndrome to reduce the diagnostic delay and increase patients’ access to treatment,” he said. “We hope to reach primary care, ER, and urgent care providers who are on the frontlines interacting with CVS patients seeking care, especially during an attack.”
The update was published online in Gastroenterology.
Understanding Cyclic Vomiting Syndrome
CVS is a chronic disorder of gut-brain interaction (DGBI), which is characterized by acute episodes of nausea and vomiting, separated by time without symptoms. Patients can usually identify a pattern of symptoms that show up during and between episodes.
CVS can vary, ranging from mild — with less than four episodes per year and lasting less than 2 days — to moderate-severe — with more than four episodes per year, lasting more than 2 days, and requiring at least one emergency department visit or hospitalization.
The disorder has four distinct phases — inter-episodic, prodromal, emetic, and recovery — that align with distinct treatment and management strategies. Between episodes, patients typically don’t experience repetitive vomiting but may experience symptoms such as mild nausea, indigestion, and occasional vomiting. Although CVS episodes can happen at any time, most tend to occur in the early morning.
For diagnosis, clinicians should consider CVS in adults presenting with episodic bouts of repetitive vomiting, following criteria established by the Rome Foundation. Rome IV criteria include acute-onset vomiting lasting less than 7 days, at least three discrete episodes in a year with two in the previous 6 months, and an absence of vomiting between episodes separated by at least 1 week of baseline health.
About 65% of patients with CVS experience prodromal symptoms, which last for about an hour before the onset of vomiting and may include panic, a sense of doom, and an inability to communicate effectively. During prodromal or emetic phases, patients have also reported fatigue, brain fog, restlessness, anxiety, headache, bowel urgency, abdominal pain, flushing, or shakiness.
As with migraines, CVS episodes may often be triggered by psychological and physiological factors, particularly stress. Episodes can stem from both negative stress, such as a death or relationship conflicts, as well as positive stress, such as birthdays and vacations. Other triggers include sleep deprivation, hormonal fluctuations linked to the menstrual cycle, travel, motion sickness, or acute infections.
Adult CVS is associated with several conditions, particularly mood disorders, including anxiety, depression, and panic disorder. Patients may also experience migraines, seizure disorders, or autonomic imbalances, such as postural orthostatic tachycardia syndrome, which may indicate pathophysiological mechanisms and routes for management.
The American Neurogastroenterology and Motility Society recommends testing to rule out similar or overlapping conditions, such as Addison’s disease, hypothyroidism, and hepatic porphyria. Diagnostic workup should include blood work, urinalysis, and one-time esophagogastroduodenoscopy or upper gastrointestinal imaging. Repeated imaging and gastric emptying scans should be avoided.
Providing Treatment and Prevention
For treatment, knowing the CVS phase is “essential,” the authors wrote. For instance, during the prodromal phase, abortive therapies can halt the transition to the emetic phase, and earlier intervention is associated with a higher probability of stopping an episode. The authors recommend intranasal sumatriptan, ondansetron, antihistamines, and sedatives.
During the emetic phase, supportive therapy can help terminate the episode. This may include continuing the abortive regimen and going to the emergency department for hydration and antiemetic medications. Patients may also find relief in a quiet, darker room in the emergency department, along with IV benzodiazepines, with the goal of inducing sedation.
During the recovery phase, patients should rest and focus on rehydration and nutrition to return to the well phase.
During the well or inter-episodic phase, patients can follow lifestyle measures to identify and avoid triggers, such as taking prophylactic medication (tricyclic antidepressants, anticonvulsants, and neurokinin-1 receptor antagonists such as aprepitant), reducing stress, and implementing a good sleep routine.
As part of patient education, clinicians can discuss the four phases and rehearse the actions to take to prevent or stop an episode.
“CVS has a significant impact on patients, families, and the healthcare system. The unpredictable and disruptive nature of episodes can result in reduced health-related quality of life, job loss precipitated by work absenteeism, and even divorce,” said Rosita Frazier, MD, a gastroenterologist at Mayo Clinic Arizona in Scottsdale who specializes in DGBI and CVS. Dr. Frazier, who wasn’t involved with the clinical practice update, has previously written about CVS diagnosis and management.
Patients with CVS often report negative interactions with physicians, particularly in the emergency department, where they may request specific treatments based on past experiences but are labeled as “drug seeking” and denied standard medical treatment, she said.
“Providing an individualized care plan for all patients could potentially address this problem and improve the physician-patient interaction,” she said. “Educational efforts to raise awareness among the medical community and increase both patient and provider engagement can optimize outcomes and are needed to address this critical problem.”
The authors received no specific funding for this update. Dr. Levinthal is a consultant for Takeda Pharmaceuticals and Mahana. Dr. Frazier reported no relevant financial disclosures.
, according to a new clinical practice update from the American Gastroenterological Association.
CVS affects up to 2% of U.S. adults and is more common in women, young adults, and those with a personal or family history of migraine headaches. However, most patients don’t receive a diagnosis or often experience years of delay in receiving effective treatment.
“A diagnosis is a powerful tool. Not only does it help patients make sense of debilitating symptoms, but it allows healthcare providers to create an effective treatment plan,” said author David J. Levinthal, MD, AGAF, director of the Neurogastroenterology and Motility Center at the University of Pittsburgh Medical Center.
“Our goal with this clinical practice update is to increase awareness of cyclic vomiting syndrome to reduce the diagnostic delay and increase patients’ access to treatment,” he said. “We hope to reach primary care, ER, and urgent care providers who are on the frontlines interacting with CVS patients seeking care, especially during an attack.”
The update was published online in Gastroenterology.
Understanding Cyclic Vomiting Syndrome
CVS is a chronic disorder of gut-brain interaction (DGBI), which is characterized by acute episodes of nausea and vomiting, separated by time without symptoms. Patients can usually identify a pattern of symptoms that show up during and between episodes.
CVS can vary, ranging from mild — with less than four episodes per year and lasting less than 2 days — to moderate-severe — with more than four episodes per year, lasting more than 2 days, and requiring at least one emergency department visit or hospitalization.
The disorder has four distinct phases — inter-episodic, prodromal, emetic, and recovery — that align with distinct treatment and management strategies. Between episodes, patients typically don’t experience repetitive vomiting but may experience symptoms such as mild nausea, indigestion, and occasional vomiting. Although CVS episodes can happen at any time, most tend to occur in the early morning.
For diagnosis, clinicians should consider CVS in adults presenting with episodic bouts of repetitive vomiting, following criteria established by the Rome Foundation. Rome IV criteria include acute-onset vomiting lasting less than 7 days, at least three discrete episodes in a year with two in the previous 6 months, and an absence of vomiting between episodes separated by at least 1 week of baseline health.
About 65% of patients with CVS experience prodromal symptoms, which last for about an hour before the onset of vomiting and may include panic, a sense of doom, and an inability to communicate effectively. During prodromal or emetic phases, patients have also reported fatigue, brain fog, restlessness, anxiety, headache, bowel urgency, abdominal pain, flushing, or shakiness.
As with migraines, CVS episodes may often be triggered by psychological and physiological factors, particularly stress. Episodes can stem from both negative stress, such as a death or relationship conflicts, as well as positive stress, such as birthdays and vacations. Other triggers include sleep deprivation, hormonal fluctuations linked to the menstrual cycle, travel, motion sickness, or acute infections.
Adult CVS is associated with several conditions, particularly mood disorders, including anxiety, depression, and panic disorder. Patients may also experience migraines, seizure disorders, or autonomic imbalances, such as postural orthostatic tachycardia syndrome, which may indicate pathophysiological mechanisms and routes for management.
The American Neurogastroenterology and Motility Society recommends testing to rule out similar or overlapping conditions, such as Addison’s disease, hypothyroidism, and hepatic porphyria. Diagnostic workup should include blood work, urinalysis, and one-time esophagogastroduodenoscopy or upper gastrointestinal imaging. Repeated imaging and gastric emptying scans should be avoided.
Providing Treatment and Prevention
For treatment, knowing the CVS phase is “essential,” the authors wrote. For instance, during the prodromal phase, abortive therapies can halt the transition to the emetic phase, and earlier intervention is associated with a higher probability of stopping an episode. The authors recommend intranasal sumatriptan, ondansetron, antihistamines, and sedatives.
During the emetic phase, supportive therapy can help terminate the episode. This may include continuing the abortive regimen and going to the emergency department for hydration and antiemetic medications. Patients may also find relief in a quiet, darker room in the emergency department, along with IV benzodiazepines, with the goal of inducing sedation.
During the recovery phase, patients should rest and focus on rehydration and nutrition to return to the well phase.
During the well or inter-episodic phase, patients can follow lifestyle measures to identify and avoid triggers, such as taking prophylactic medication (tricyclic antidepressants, anticonvulsants, and neurokinin-1 receptor antagonists such as aprepitant), reducing stress, and implementing a good sleep routine.
As part of patient education, clinicians can discuss the four phases and rehearse the actions to take to prevent or stop an episode.
“CVS has a significant impact on patients, families, and the healthcare system. The unpredictable and disruptive nature of episodes can result in reduced health-related quality of life, job loss precipitated by work absenteeism, and even divorce,” said Rosita Frazier, MD, a gastroenterologist at Mayo Clinic Arizona in Scottsdale who specializes in DGBI and CVS. Dr. Frazier, who wasn’t involved with the clinical practice update, has previously written about CVS diagnosis and management.
Patients with CVS often report negative interactions with physicians, particularly in the emergency department, where they may request specific treatments based on past experiences but are labeled as “drug seeking” and denied standard medical treatment, she said.
“Providing an individualized care plan for all patients could potentially address this problem and improve the physician-patient interaction,” she said. “Educational efforts to raise awareness among the medical community and increase both patient and provider engagement can optimize outcomes and are needed to address this critical problem.”
The authors received no specific funding for this update. Dr. Levinthal is a consultant for Takeda Pharmaceuticals and Mahana. Dr. Frazier reported no relevant financial disclosures.
FROM GASTROENTEROLOGY
FDA OKs Voquezna for Heartburn Relief in Nonerosive Gastroesophageal Reflux Disease
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
It represents the third indication for the potassium-competitive acid blocker, which is already approved to treat all severities of erosive esophagitis and to eradicate Helicobacter pylori infection in combination with antibiotics.
The approval in nonerosive GERD was supported by results of the PHALCON-nonerosive GERD-301 study, a phase 3 randomized, placebo-controlled, double-blind, multicenter study evaluating the safety and efficacy of once-daily Voquezna in more than 700 adults with nonerosive GERD experiencing at least 4 days of heartburn per week.
“Vonoprazan was efficacious in reducing heartburn symptoms in patients with [nonerosive GERD], with the benefit appearing to begin as early as the first day of therapy. This treatment effect persisted after the initial 4-week placebo-controlled period throughout the 20-week extension period,” the study team wrote in a paper published online in Clinical Gastroenterology and Hepatology , and reported on by this news organization.
Voquezna “provides physicians with a novel, first-in-class treatment that can quickly and significantly reduce heartburn for many adult patients” with nonerosive GERD, Colin W. Howden, MD, AGAF, professor emeritus, University of Tennessee College of Medicine in Memphis, said in a news release.
The most common adverse events reported in patients treated with Voquezna during the 4-week placebo-controlled period were abdominal pain, constipation, diarrhea, nausea, and urinary tract infection.
Upper respiratory tract infection and sinusitis were also reported in patients who taking Voquezna in the 20-week extension phase of the trial.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
Which GI Side Effects Should GLP-1 Prescribers Worry About?
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Esophageal Cancer Risk Unchanged After Helicobacter Eradication
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Eosinophilic Esophagitis Often Persists Despite Treatment
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
FROM GASTRO HEP ADVANCES
Dupilumab Effective in PPI-Refractory Pediatric EoE
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
GLP-1 Receptor Agonists in Endoscopy
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.

























