User login
Mass shooters and mental illness: Reexamining the connection
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Delirious mania: Presentation, pathogenesis, and management
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
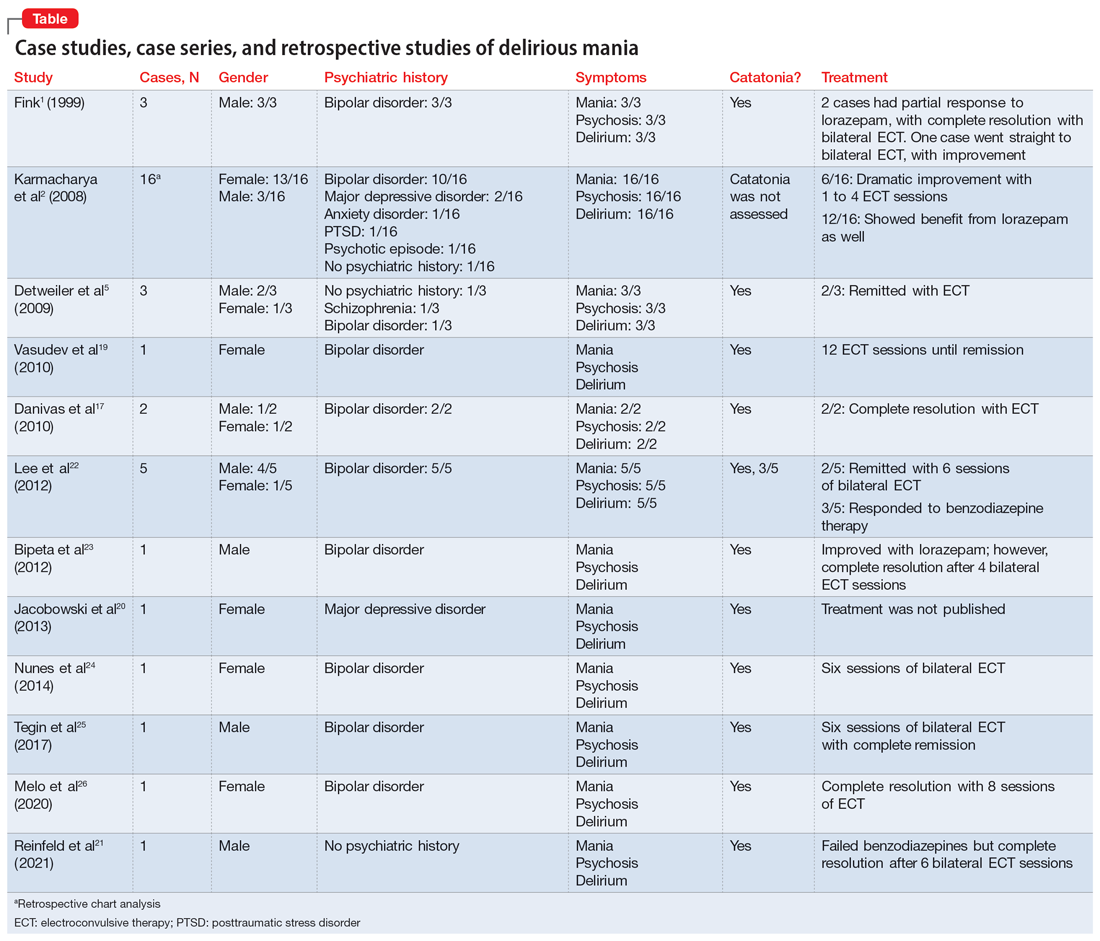
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).
Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
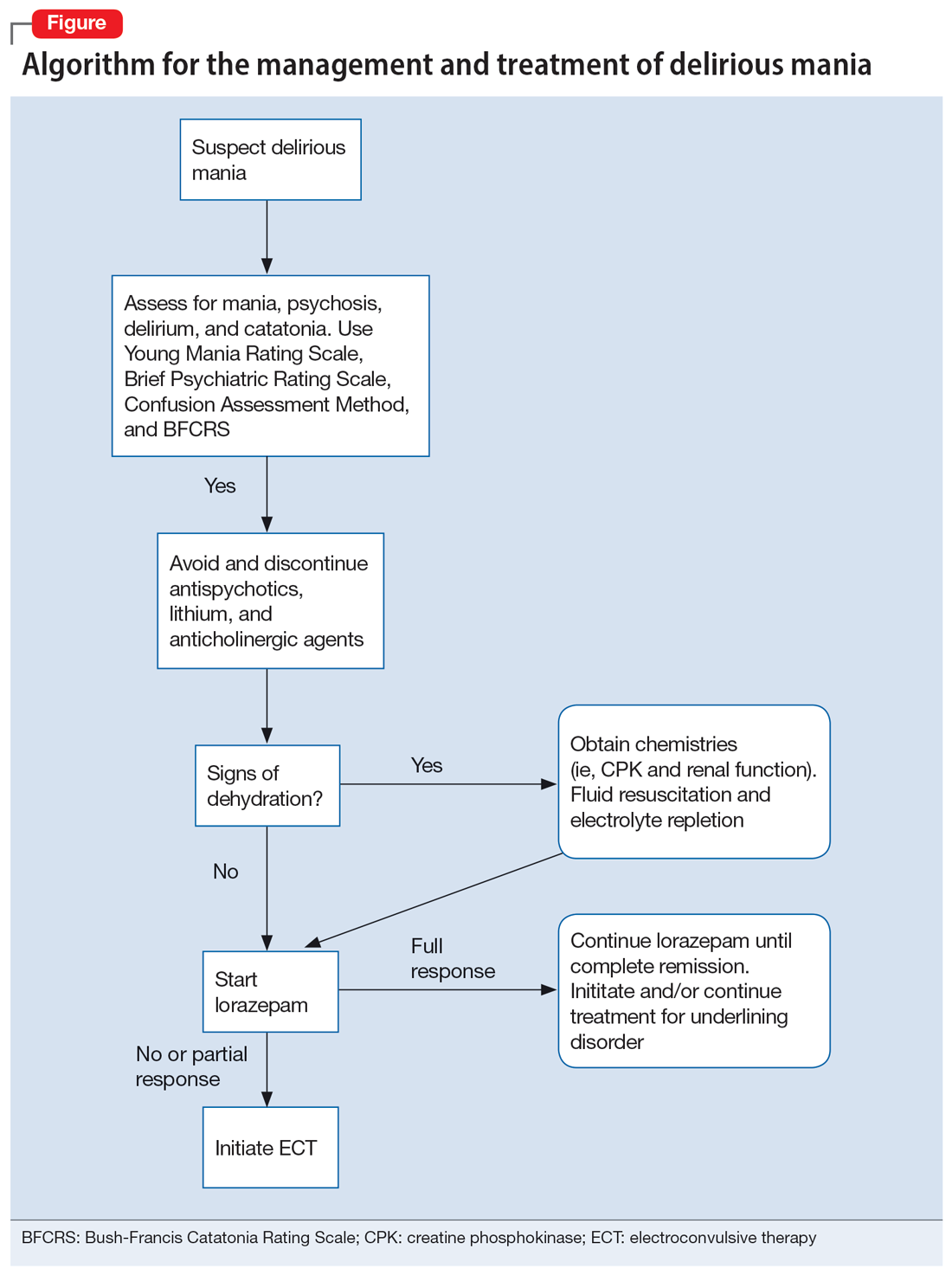
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.
Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Worsening mania while receiving low-dose quetiapine: A case report
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1Aantagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Bipolar disorder may raise risk of polycystic ovarian syndrome
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Previous studies suggest that the prevalence of polycystic ovarian syndrome (PCOS) is higher in bipolar disorder (BD) patients compared with individuals not diagnosed with BD, wrote Jieyu Liu, PhD, of the Second Xiangya Hospital of Central South University, Hunan, China, and colleagues.
However, studies have been limited to drug-treated BD patients, and data on the effects of BD on the development of PCOS are limited, they said. Data from previous studies also indicate that serum testosterone levels, serum androstenedione levels, and polycystic ovarian morphology (PCOM) are increased in BD patients compared with women without BD.
In a study published in the Journal of Affective Disorders, the researchers recruited 72 BD patients on long-term medication, 72 drug-naive patients, and 98 healthy controls between March 2022 and November 2022.
PCOM was assessed using ≥ 8 MHz transvaginal transducers to determine the number of follicles and ovarian volume. PCOS was then defined using the Rotterdam criteria, in which patients met two of three qualifications: oligoovulation or anovulation; hyperandrogenemia; or PCOM (excluding other endocrine diseases).
In a multivariate analysis, drug-naive women with BD had significantly higher rates of PCOS compared with healthy controls (odds ratio 3.02). The drug-naive BD patients also had a greater prevalence of oligoamenorrhea compared with healthy controls (36.36% vs. 12.12%) and higher levels of anti-mullerian hormone, luteinizing hormone, and follicle stimulating hormone compared to the controls.
A further regression analysis showed that those on long-term valproate treatment had the highest risk (OR 3.89) and the prevalence of PCOS was significantly higher among patients treated with valproate compared with drug-naive patients (53.3% vs. 30.6%). Younger age and the presence of insulin resistance also were associated with increased risk of PCOS (OR 0.37 and OR 1.73, respectively).
“Unexpectedly, no significant differences in serum androgen levels, including TT, FAI, androstenedione, and [dehydroepiandrosterone sulfate] levels, were observed between drug-naive BD patients and the HCs,” the researchers wrote in their discussion. This difference may stem from multiple causes including demographic variables, inclusion of PCOM as a diagnostic criterion, and the impact of genetic and environmental factors, they said.
The findings were limited by several factors including the small study population, which prevented conclusions of causality and comparison of the effects of different mood stabilizers on PCOS, the researchers noted. Other limitations included the relatively homogeneous population from a single region in China, and the inability to account for the effects of diet and lifestyle.
More research is needed to explore the impact of mediations, but the results suggest that BD patients are susceptible to PCOS; therefore, they should evaluate their reproductive health before starting any medication, and review reproductive health regularly, the researchers concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Perinatal depression rarely stands alone
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
A blood test to diagnose bipolar disorder?
TOPLINE:
(MDD), new research shows. Investigators state that the test could identify up to 30% of patients with BD when used on its own and could be even more effective when combined with a standardized psychometric assessment.
METHODOLOGY:
- In the proof-of-concept study, investigators sought to identify biomarkers to accurately identify BD, which is frequently misdiagnosed as MDD because of overlapping symptoms and the lack of objective diagnostic tools.
- The study included 241 participants (70% female; mean age, 28 years) from the U.K.-based Delta Study who had been diagnosed with MDD within the past 5 years and had depressive symptoms as assessed with the Patient Health Questionnaire-9 (score ≥ 5).
- Participants completed an online questionnaire that included questions from the Mood Disorder Questionnaire and the Warwick-Edinburgh Mental Well-Being Scale and were asked to return a dried blood spot (DBS) fasting blood sample.
- Investigators analyzed the DBS samples for 630 metabolites and contacted participants by phone to establish diagnoses at 6 and 12 months using the World Health Organization World Mental Health Composite International Diagnostic Interview.
TAKEAWAY:
- Investigators used a panel of 17 biomarkers to correctly identify 67 (27.8%) participants with BD who had been previously misdiagnosed with MDD. They confirmed MDD in the remaining 174 patients.
- The biomarkers used in the test were correlated primarily with lifetime manic symptoms and were validated in a separate group of 30 patients.
- The identified biomarker panel provided a mean cross-validated area under the receiver operating characteristic curve of 0.71 (P < .001), with ceramide d18:0/24:1 emerging as the strongest biomarker.
- Combining biomarker readouts with patient-reported data significantly improved the performance of diagnostic models based on extensive demographic data and information from the Patient Health Questionnaire and Mood Disorder Questionnaire (P = .03 for all).
IN PRACTICE:
“The added value of biomarkers was particularly evident in scenarios where data on psychiatric symptoms were unavailable and at intermediate diagnostic thresholds, suggesting that biomarker tests may especially benefit patients who do not report their symptoms and whose diagnoses are uncertain,” the authors write.
SOURCE:
Jakub Tomasik, PhD, of the University of Cambridge (England), led the study, which was published online in JAMA Psychiatry. Stanley Medical Research Institute and Psyomics funded the study.
LIMITATIONS:
Data on confounding factors such as diet and blood pressure were missing. In addition, investigators noted that the sample mostly comprised White Internet users and was not representative of all individuals with BD.
Dr. Tomasik has a patent pending for DBS blood biomarkers. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MDD), new research shows. Investigators state that the test could identify up to 30% of patients with BD when used on its own and could be even more effective when combined with a standardized psychometric assessment.
METHODOLOGY:
- In the proof-of-concept study, investigators sought to identify biomarkers to accurately identify BD, which is frequently misdiagnosed as MDD because of overlapping symptoms and the lack of objective diagnostic tools.
- The study included 241 participants (70% female; mean age, 28 years) from the U.K.-based Delta Study who had been diagnosed with MDD within the past 5 years and had depressive symptoms as assessed with the Patient Health Questionnaire-9 (score ≥ 5).
- Participants completed an online questionnaire that included questions from the Mood Disorder Questionnaire and the Warwick-Edinburgh Mental Well-Being Scale and were asked to return a dried blood spot (DBS) fasting blood sample.
- Investigators analyzed the DBS samples for 630 metabolites and contacted participants by phone to establish diagnoses at 6 and 12 months using the World Health Organization World Mental Health Composite International Diagnostic Interview.
TAKEAWAY:
- Investigators used a panel of 17 biomarkers to correctly identify 67 (27.8%) participants with BD who had been previously misdiagnosed with MDD. They confirmed MDD in the remaining 174 patients.
- The biomarkers used in the test were correlated primarily with lifetime manic symptoms and were validated in a separate group of 30 patients.
- The identified biomarker panel provided a mean cross-validated area under the receiver operating characteristic curve of 0.71 (P < .001), with ceramide d18:0/24:1 emerging as the strongest biomarker.
- Combining biomarker readouts with patient-reported data significantly improved the performance of diagnostic models based on extensive demographic data and information from the Patient Health Questionnaire and Mood Disorder Questionnaire (P = .03 for all).
IN PRACTICE:
“The added value of biomarkers was particularly evident in scenarios where data on psychiatric symptoms were unavailable and at intermediate diagnostic thresholds, suggesting that biomarker tests may especially benefit patients who do not report their symptoms and whose diagnoses are uncertain,” the authors write.
SOURCE:
Jakub Tomasik, PhD, of the University of Cambridge (England), led the study, which was published online in JAMA Psychiatry. Stanley Medical Research Institute and Psyomics funded the study.
LIMITATIONS:
Data on confounding factors such as diet and blood pressure were missing. In addition, investigators noted that the sample mostly comprised White Internet users and was not representative of all individuals with BD.
Dr. Tomasik has a patent pending for DBS blood biomarkers. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MDD), new research shows. Investigators state that the test could identify up to 30% of patients with BD when used on its own and could be even more effective when combined with a standardized psychometric assessment.
METHODOLOGY:
- In the proof-of-concept study, investigators sought to identify biomarkers to accurately identify BD, which is frequently misdiagnosed as MDD because of overlapping symptoms and the lack of objective diagnostic tools.
- The study included 241 participants (70% female; mean age, 28 years) from the U.K.-based Delta Study who had been diagnosed with MDD within the past 5 years and had depressive symptoms as assessed with the Patient Health Questionnaire-9 (score ≥ 5).
- Participants completed an online questionnaire that included questions from the Mood Disorder Questionnaire and the Warwick-Edinburgh Mental Well-Being Scale and were asked to return a dried blood spot (DBS) fasting blood sample.
- Investigators analyzed the DBS samples for 630 metabolites and contacted participants by phone to establish diagnoses at 6 and 12 months using the World Health Organization World Mental Health Composite International Diagnostic Interview.
TAKEAWAY:
- Investigators used a panel of 17 biomarkers to correctly identify 67 (27.8%) participants with BD who had been previously misdiagnosed with MDD. They confirmed MDD in the remaining 174 patients.
- The biomarkers used in the test were correlated primarily with lifetime manic symptoms and were validated in a separate group of 30 patients.
- The identified biomarker panel provided a mean cross-validated area under the receiver operating characteristic curve of 0.71 (P < .001), with ceramide d18:0/24:1 emerging as the strongest biomarker.
- Combining biomarker readouts with patient-reported data significantly improved the performance of diagnostic models based on extensive demographic data and information from the Patient Health Questionnaire and Mood Disorder Questionnaire (P = .03 for all).
IN PRACTICE:
“The added value of biomarkers was particularly evident in scenarios where data on psychiatric symptoms were unavailable and at intermediate diagnostic thresholds, suggesting that biomarker tests may especially benefit patients who do not report their symptoms and whose diagnoses are uncertain,” the authors write.
SOURCE:
Jakub Tomasik, PhD, of the University of Cambridge (England), led the study, which was published online in JAMA Psychiatry. Stanley Medical Research Institute and Psyomics funded the study.
LIMITATIONS:
Data on confounding factors such as diet and blood pressure were missing. In addition, investigators noted that the sample mostly comprised White Internet users and was not representative of all individuals with BD.
Dr. Tomasik has a patent pending for DBS blood biomarkers. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
Pandemic-era telehealth led to fewer therapy disruptions
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
Smart bracelet may predict mood changes in bipolar disorder
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ECNP 2023
Irritable temperament predicts bipolar disorder risk
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
When German psychiatrist Emil Kraepelin (1856-1926) studied emotions in patients with affective disorders, he identified four temperaments: the depressive (DT), the hyperthymic (HT), the irritable (IT), and the cyclothymic (CT). Subsequent researchers later identified an anxious temperament (AT).
“The notion that temperaments can be useful in predicting bipolar disorders sparked a plethora of research,” wrote Elie G. Karam, MD, of Saint George Hospital, Beirut, and colleagues. In particular, the cyclothymic (CT) and irritable (IT) temperament types have been targeted in studies of patients with bipolar disorders, but previous studies of temperament and bipolar have been limited by methodological issues, they said.
In a study published in European Psychiatry, the researchers reviewed data from 1,723 consecutive adult outpatients who presented to a university-based mental health clinic with various symptoms between January 2014 and September 2019.
Patients were assessed using the Hypomania Checklist-32 (HCL-32) and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego-Auto-questionnaire (TEMPS-A), then were diagnosed by psychiatrists using DSM-5 criteria. Patients with any bipolar types as defined by the DSM-5 underwent simple and multiple binary logistic regression analyses. The analysis included continuous scores and categorical normalized z-scores.
A total of 369 patients had confirmed DSM-5 diagnosis of bipolar disorder (52 with type I, 176 with type II, 102 with other specified bipolar and related disorder, and 39 with substance- or medication-induced bipolar disorder. The mean age of the participants was 38 years, and 54% were female.
In a bivariate analysis, all continuous temperament scores were significant predictors of bipolar disorder; all except AT remained significant in multivariate analysis. Increasing scores of IT, CT, and HT were associated with bipolar disorder, but increasing scores of DT were reflective of lower chance of bipolar disorder, the researchers noted.
In multivariate analysis of categorical normalized z-scores, IT and CT were significant predictors of bipolar disorder. At the highest point, CT was the stronger predictor, compared with IT (odds ratio, 3.84 vs. 2.55); having a higher DT score significantly reduced the odds of bipolar disorder (OR, 0.50).
However, “after adjusting for the presence of all temperaments as well as age and gender, only IT remained a significant predictor of patients with bipolar I disorder with adjusted OR of 1.19,” the researchers wrote.
“Correlations among temperaments were solid whether looking at patients with bipolarity or not, further emphasizing the necessity of controlling for them,” the researchers wrote in their discussion.
The findings were limited by several factors including the lack of structured interviews, the use of an outpatient-only sample, and the small number of bipolar I patients, the researchers noted.
However, the result suggest that IT can serve as a predictor of bipolar I and bipolar II disorders they said. Given the underdiagnosis of bipolar disorder in many studies, the incorporation of temperaments into the assessment of patients and research participants alike is likely to help us detect the presence of bipolarity more readily and quite importantly help us in our quest to understand their genesis,” they concluded.
The study was supported in part by anonymous private unrestricted donations to IDRAAC, Lebanon, and by Eli Lilly. The researchers had no financial conflicts to disclose.
FROM EUROPEAN PSYCHIATRY
Dialectical behavior therapy decreased suicide attempts in bipolar teens
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
, based on data from 100 individuals aged 12-18 years.
Bipolar spectrum disorder (BP) is known to substantially increase the risk for suicide in youth, but no psychosocial intervention for this population has targeted suicidal behavior in particular, wrote Tina R. Goldstein, PhD, of the University of Pittsburgh, and colleagues.
Dialectical behavior therapy (DBT) had shown effectiveness for decreasing suicide attempts in adults with borderline personality disorder, and previous studies of DBT have shown reduced suicidal ideation, self-harm, and suicide attempts in suicidal adolescents, but these studies have mainly excluded BP teens, the researchers said.
In a study published in JAMA Psychiatry, the researchers recruited adolescents aged 12-18 years with a diagnosis of BP who were treated at an outpatient clinic between November 2014 and September 2019. Of these, 47 were randomized to 1 year of DBT (a total of 36 sessions) and 53 to standard of care (SOC) psychotherapy. All participants also received medication using a flexible algorithm.
The primary outcomes were suicide attempts over a 1-year period and measurements of mood symptoms and states, specifically depression and hypomania/mania. Secondary analyses included the effect of DBT on individuals with a history of suicide attempt and on improving emotion dysregulation. The mean age of the participants was 16.1 years; 85 were female, and 74% were White.
Participants in both DBT and SOC groups reported similar rates of suicide attempt rates at study enrollment based on the Adolescent Longitudinal Follow-Up Evaluation (ALIFE) with a mean of 2.0 and 1.8 attempts, respectively (P = .80). Based on the Columbia–Suicide Severity Rating Scale Pediatric Version (C-SSRS), participants in the DBT group had slightly more suicide attempts than the SOC group at study enrollment, with a mean of 1.4 and 0.6 attempts, respectively (P = .02).
Controlling for baseline attempts, participants in the DBT group had significantly fewer suicide attempts over the study period, compared with the SOC group as measured by both ALIFE (mean 0.2 vs. 1.1) and C-SSRS (mean 0.04 vs. 0.10, P = .03 for both measures). The incidence rate ratios for reduced suicide attempts were 0.32 for ALIFE and 0.13 for C-SSRS, both significant in favor of DBT, compared with SOC.
Overall, both groups showed similarly significant improvement on measures of mood symptoms and episodes over the study period. The standardized depression rating scale slope was –0.17 and the standardized mania rating scale slope was –0.24.
DBT was significantly more effective than SOC psychotherapy at decreasing suicide attempts over 1 year (ALIFE: incidence rate ratio, 0.32; 95% CI, 0.11-0.96; C-SSRS: IRR, 0.13; 95% CI, 0.02-0.78).
On further analysis, the decrease in suicide attempts in the DBT group was greater over time and among those with a lifetime history of suicide attempts (IRR, 0.23). “Decreased risk of suicide attempt in DBT was mediated by improvement in emotion dysregulation, particularly for those with high baseline emotion dysregulation,” the researchers wrote in their discussion.
The findings were limited by several factors including the mainly female, non-Hispanic White study population, and controlled clinical setting, the researchers noted. Data from a forthcoming community implementation field trial will address some generalizability issues, although more work is needed to address disparities in BP diagnosis and treatment, they added.
However, the results support the potential of DBT for mood management and for reducing suicide attempts in a high-risk adolescent population, especially those with high levels of emotional dysregulation, on par with other established psychosocial treatments, the researchers concluded.
More options needed to manage increased risk
“It was important to conduct this study at this time because, while still relatively rare, bipolar spectrum disorders in adolescents confer increased risk for suicide,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. The complexity of BP and the increased risk of suicide in these patients challenge clinicians to identify robust evidence-based interventions beyond pharmacotherapy that mitigate this risk, said Dr. Loper, who is triple board certified in pediatrics, general psychiatry, and child & adolescent psychiatry, but was not involved in the study.
The current study findings were not surprising, because DBT has proven effective in decreasing suicidal ideation and suicide attempts in other high-risk adolescent patient populations, Dr. Loper said. “Given the therapeutic content of DBT, with emphasis on mindfulness, distress tolerance, social skills, and emotional regulation, I think it is reasonable to hypothesize that DBT might be a globally applicable intervention, independent of mental health diagnosis or etiology of suicidal ideation,” he said.
The take-home message for clinicians is that the results support the efficacy of DBT as an intervention for adolescents with BP and suicidal ideation, self-injurious behavior, or suicide attempts, said Dr. Loper. For these patients, given their increased suicide risk, “DBT should certainly be recommended as a component of their treatment plan,” he said.
However, barriers to the use of DBT in clinical practice exist, notably access and cost, Dr. Loper noted. “I think that the most prominent barrier in accessing DBT in clinical practice is the availability of certified, structured DBT treatment programs, and particularly those willing to provide services to adolescents,” he said. “Additionally, certified DBT programs, which are the gold standard, are often not covered by third-party payers, making cost yet another potential barrier.”
Looking ahead, Dr. Loper agreed with the study authors that additional research with a more diverse patient population representative of adolescents with bipolar spectrum disorder “is a crucial area of focus.”
The study was funded by the National Institutes of Mental Health through a grant to Dr. Goldstein, who also disclosed royalties from Guilford Press unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
FROM JAMA PSYCHIATRY









