User login
Managing maternal mortality with multifetal pregnancy reduction
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
Left upper quadrant entry is often a reliable alternative to umbilicus
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.
The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.
However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
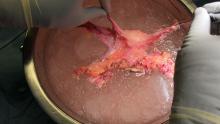
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
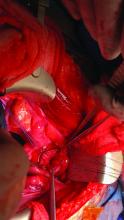
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
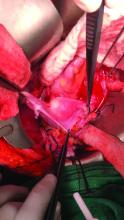
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.
The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
The choice of entry point for gynecologic laparoscopy is critical, considering that most laparoscopic injuries occur during initial entry into the abdomen. In addition, different abdominal access points may have differing utility and efficacy depending on the patient. (The overall rate of injuries to abdominal viscera and blood vessels at the time of entry is an estimated 1 per 1,000 cases.1)
The most conventional entry point for gynecologic laparoscopic surgeries has been the umbilicus, but there are contraindications to this choice and situations in which it may not be the best access site. It is important to have knowledge of alternate entry points and techniques that consider the patient’s current pathology, anatomy, and most importantly, surgical history to better facilitate a safe initial entry.
The left upper quadrant (LUQ) has been described as a preferred alternate site to the umbilicus, and some gynecologic surgeons even consider it as a routine mode of entry.2 In our practice, LUQ entry is a safe and commonly used technique that is chosen primarily based on a patient’s history of a midline vertical incision, the presence of abdominal mesh from a prior umbilical hernia repair, or repeated cesarean sections.
Our technique for LUQ entry is a modification of the traditional approach that employs Palmer’s point – the entry point described by Raoul Palmer, MD, in 1974 as 3-4 cm below the left subcostal margin at the midclavicular line.3 We choose to enter at the midclavicular level and directly under the last rib.
When the umbilicus is problematic
The umbilicus is a favored entry point not only for its operative access to pelvic structures but also because – in the absence of obesity – it has no or little subcutaneous fat and, therefore, provides the shortest distance from skin to peritoneum.

However, adhesive disease from a prior laparotomy involving the umbilicus is a risk factor for bowel injury during umbilical entry (direct trocar, Veress needle, or open technique). In a 1995 review of 360 women undergoing operative laparoscopy after a previous laparotomy, Brill et al. reported umbilical adhesions in 27% of those with prior horizontal suprapubic (Pfannenstiel) incisions, in 55% of those with prior incisions in the midline below the umbilicus, and 67% of those with prior midline incisions above the umbilicus.4
Of the 259 patients whose prior laparotomy was for gynecologic surgery (as opposed to obstetric or general surgery) adhesions were present in 70% of those who had midline incisions. (Direct injury to adherent omentum and bowel occurred during laparoscopic procedures in 21% of all women.)
Since the Brill paper, other studies have similarly reported significant adhesion rate, especially after midline incisions. For instance, one French study of patients undergoing laparoscopy reported umbilical adhesions in 51.7% of 89 patients who had previous laparotomy with a midline incision.5
Prior umbilical laparoscopy is not a risk factor for umbilical entry unless a hernia repair with mesh was performed at the umbilicus. Umbilical adhesions have been reported to occur in up to 15% of women who have had prior laparoscopic surgery, with more adhesions associated with larger trocar use (specifically 12-mm trocars).1 Still, the rate of those adhesions was very low.
Obesity is not necessarily a contraindication to umbilical entry; however, it can make successful entry more difficult, particularly in those with central obesity and a thicker layer of subcutaneous fat. It can be difficult in such cases to know when peritoneal access is achieved. Extra-long Veress needles or trocars may be needed, and it is important to enter the abdomen at a 90° angle to minimize risk to the great vessel vasculature.
LUQ entry is often a reliable alternative when central obesity is significant or when umbilical access proves to be difficult. Certainly, the subcutaneous fat layer is thinner at the LUQ than at the umbilicus, and in patients whose umbilicus is pulled very caudal because of a large pannus, the LUQ will also provide a better location for visualization of pelvic anatomy and for easier entry.
We still use umbilical entry in most patients with obesity, but if we are unsuccessful after two to three attempts, we proceed to the LUQ (barring any contraindications to this site).
LUQ entry: Our approach, contraindications
By entering at the midclavicular level and directly under the bottom of the rib cage, rather than 2-3 cm below the last rib as in traditional Palmer’s point LUQ entry, we benefit from the tenting up of the peritoneum by the last rib. Having space between the peritoneum and underlying omentum and stomach can facilitate an easier entry, as shown in the video.
We primarily utilize the Veress needle for entry. The needle is inserted directly perpendicular to the fascia, or at a slight angle toward the umbilicus. After the abdomen is insufflated to 15 mm Hg, we proceed with a visual peritoneal entry using a 5-mm trocar with a clear tip, which allows us to visualize both layers of fascia, and subsequently the peritoneum, as the trocar is advanced.
The fascia is not fused, so we can expect to feel three “pops” as the needle (or trocar) passes through the aponeuroses of the internal and external obliques, the aponeuroses of the internal oblique and transversus, and the peritoneum.
While successful peritoneal entry with umbilical access is generally confirmed with an intraperitoneal pressure measuring less than 7 mm Hg (which varies depending on abdominal wall thickness and adiposity), we have found that the opening pressure with LUQ entry is slightly higher. A recently published Canadian guideline for gynecologic laparoscopic entry recommends that an initial Veress intraperitoneal pressure of 10 mm Hg or below be considered an indicator of successful entry, regardless of the patient’s body habitus.1
LUQ entry can be helpful for surgeries involving large pelvic masses, for which there is little or no space to enter at the umbilicus or to optimally view the pathology. Utilizing the LUQ not only allows for an unobstructed entry and optimal viewing but also may become an extra operative port that can be used for the camera, allowing both surgeons to operate with two hands – a four-port technique. It also allows the surgeon to use a larger diameter port at the umbilicus without concern for cosmetics.
Additionally, there is a school of thought that LUQ entry is overall more successful, requiring less conversion to alternative sites and fewer attempts. This success may result from the presence of less adhesive disease in the LUQ, as well as clearer visualization of the anatomy while entering and confidence in entering the intraperitoneal space.
A prerequisite for LUQ entry is that the stomach be decompressed through placement of an oral gastric or nasogastric tube and suctioning of all gastric contents. An inability to decompress the stomach is a contraindication to LUQ entry, as is a history of splenectomy, an enlarged liver, gastric bypass surgery, or upper abdominal surgery.
Entry techniques, alternate sites
No single entry site or technique has been proven to be universally safer than another. A 2019 Cochrane review of laparoscopic entry techniques noted an advantage of direct trocar entry over Veress-needle entry for failed entry but concluded that, overall, evidence was insufficient to support the use of one entry technique over another to decrease complication rates.6
A more recently published review of randomized controlled trials, Cochrane reviews, and older descriptive accounts similarly concluded that, between the Veress needle (the oldest described technique), direct trocar insertion, and open entry (Hasson), there is no good evidence to suggest that any of these methods is universally superior.2 Surgeon comfort is, therefore, an important factor.
Regarding entry sites, we advocate use of the LUQ as an advantageous alternative site for access, but there are several other approaches described in the literature. These include right upper quadrant entry; the Lee Huang point, which is about 10 cm below the xiphoid; and uncommonly, vaginal, either posterior to the uterus into the pouch of Douglas or through the uterine fundus.2
The right upper quadrant approach is included in a recent video review in the Journal of Minimally Invasive Gynecology of safe entry techniques, along with umbilicus, LUQ, and supraumbilical entry.7
Another described entry site is the “Jain point,” located at the intersection of a vertical line drawn 2.5 cm medial to the anterior superior iliac spine, up to the level of the umbilicus, and a horizontal line at the upper margin of the umbilicus. In a retrospective study of 7,802 cases involving this method, the authors reported only one significant entry complication. Patients in the study had a wide range of BMIs and previous surgeries.8
With respect to entry techniques, we facilitate the Veress entry technique described by Frank E. Loeffler, MD, in the mid-1970s, unless there are contraindications such as second-trimester pregnancy. For umbilical entry, we first use a Kocher clamp to grasp the base of the umbilicus and then evert it. Using two towel clips, the surgeon and assistant apply countertraction by grasping the skin and fat on either side of the umbilicus. A horizontal incision is then made directly on the base of the umbilicus. The towel clips are used to elevate the anterior abdominal wall, and the Veress needle is attached to insufflation tubing, then inserted into the abdomen.
Alternatively, direct entry involves incising the skin, placing a laparoscope in a visual entry trocar, and directly visualizing each layer as the abdomen is entered. Once the trocar is intraperitoneal, insufflation is started.
In open laparoscopic/Hasson entry, the umbilical skin is incised, and the subcutaneous fat is dissected down until the rectal fascia is visualized. The fascia is then incised, the peritoneum is entered bluntly, and the Hasson trocar is placed. Insufflation is attached, and the laparoscope is inserted.
Dr. Sasaki is a partner, and Dr. McKenna is an AAGL MIGS fellow, in the private practice of Charles E. Miller, MD, & Associates in Chicago. They reported that they have no disclosures.
References
1. Vilos GA et al. J Obstet Gyneacol Can. 2021;43(3):376-89.
2. Recknagel JD and Goodman LR. J Minim Invasive Gynecol. 2021;28(3):467-74.
3. Palmer R. J Reprod Med. 1974;13:1-5.
4. Brill AI et al. Obstet Gynecol. 1995;85(2):269-72.
5. Audebert AJ and Gomel V. Fertil Steril. 2000;73(3):631-5.
6. Ahmad G et al. Cochrane Database of Systematic Reviews. 2019;1:CD006583.
7. Patzkowsky KE et al. J. Minim Invasive Gynecol. 2021;28(3):386.
8. Nutan J et al. Updates in Surgery. 2021;73(6):2321-9.
Safe abdominal laparoscopic entry
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
There are few procedures in gynecologic surgery that are blind. We can readily name dilatation and uterine curettage, but even the dreaded suction curettage can be performed under ultrasound guidance. Laparoscopy with direct insertion or with use of a Veress needle remain two of the few blind procedures in our specialty.
The reality that we all face as minimally invasive gynecologic surgeons is that, as Javier F. Magrina, MD, showed in 2002, more than 50% of injuries to the gastrointestinal tract and major blood vessels occur at entry, prior to the start of the intended surgery, with the majority occurring at the time of the primary umbilical trocar placement. In his study of over 1.5 million gynecologic patients, Dr. Magrina also noted that 20% to 25% of complications were not recognized until the postoperative period.
Interestingly, while some have recommended the open Hasson technique pioneered by Harrith M. Hasson, MD, over the blind Veress needle or direct insertion, there is no evidence to suggest it is safer. Use of shielded trocars have not been shown to decrease entry injuries; that is, visceral or vascular injuries have not been shown to decrease. Finally, at present, data do not support the recommendation that visual entry cannulas offer increased safety, although additional studies are recommended.
It is a pleasure to welcome my partner and former AAGL MIGS fellow, Kirsten J. Sasaki, MD, as well as my current AAGL MIGS fellow, Mary (Molly) McKenna, MD, to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Reference
Magrina JF. Clin Obstet Gynecol. 2002 Jun;45(2):469-80.
How 100 years of insulin have changed pregnancy for women with type 1 diabetes
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
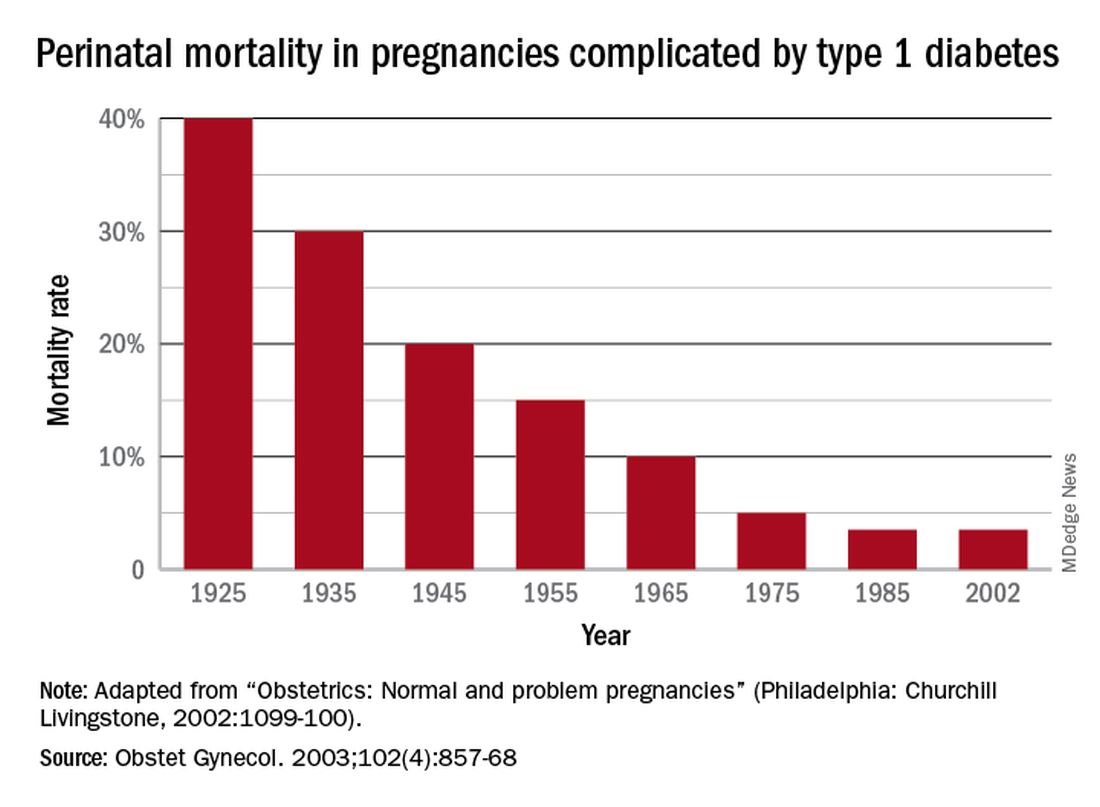
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%
In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.
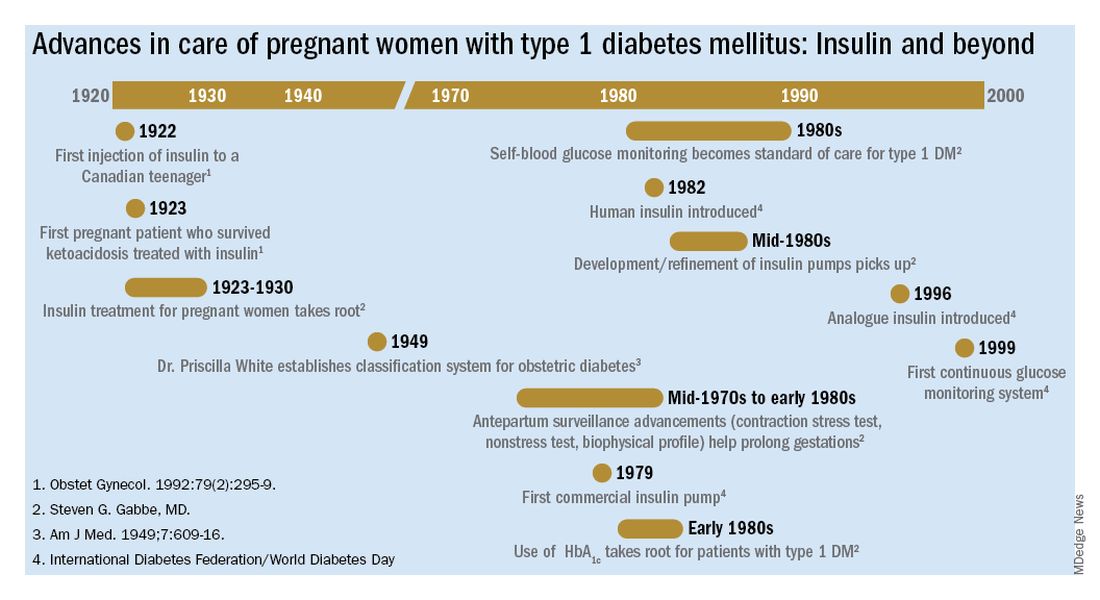
Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Insulin in pregnancy: A look back at history for Diabetes Awareness Month
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Laparoscopic abdominal cerclage: An effective, patient-sought approach for cervical insufficiency
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.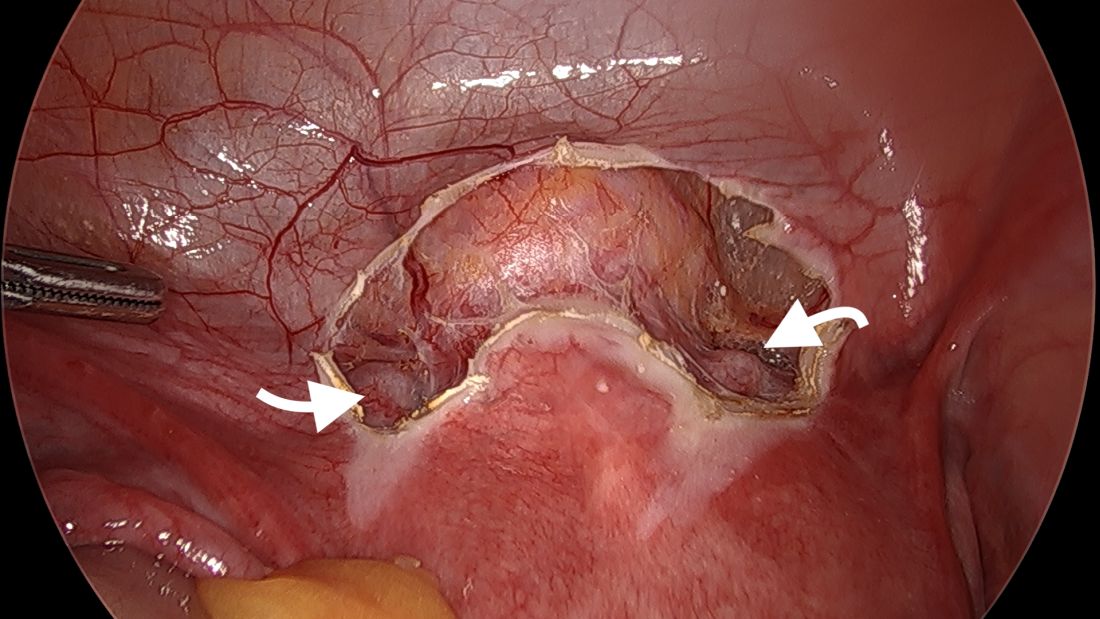
We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.
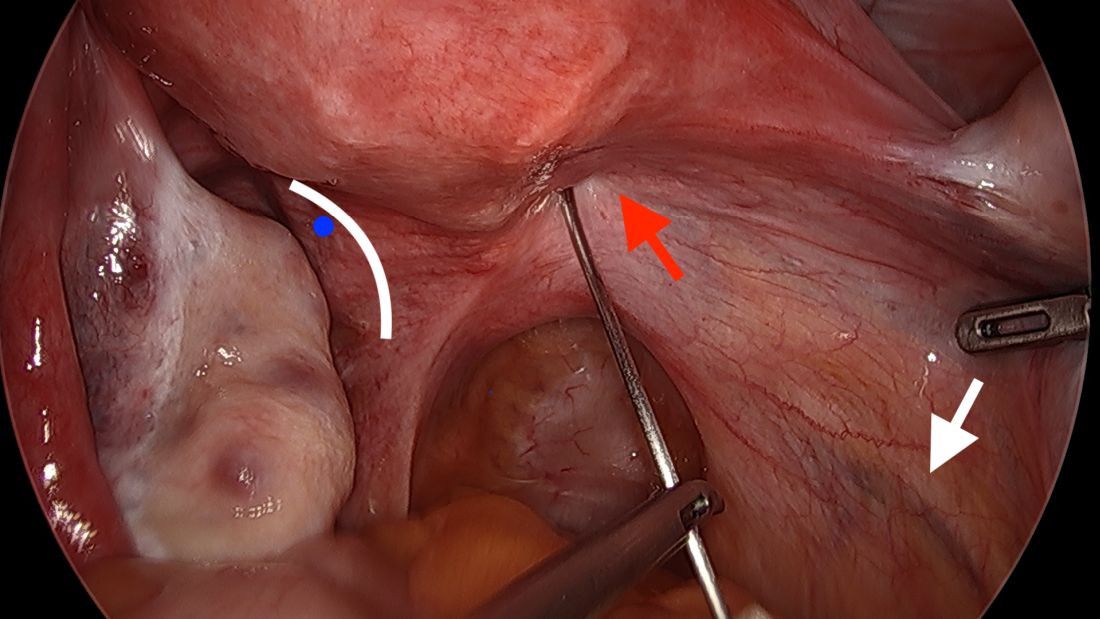
The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.
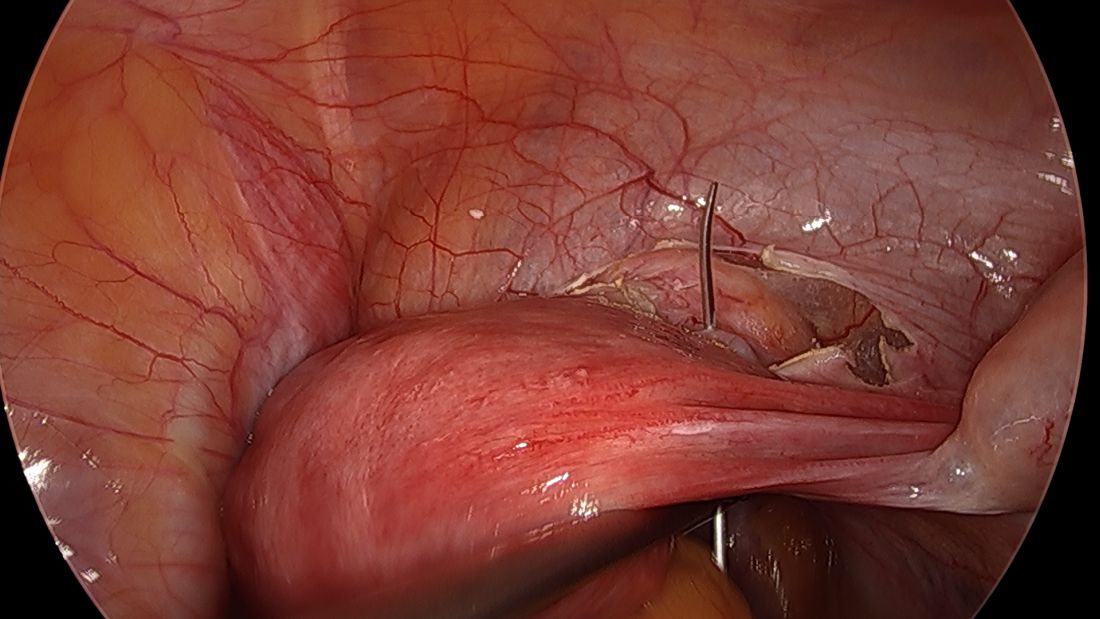
Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.
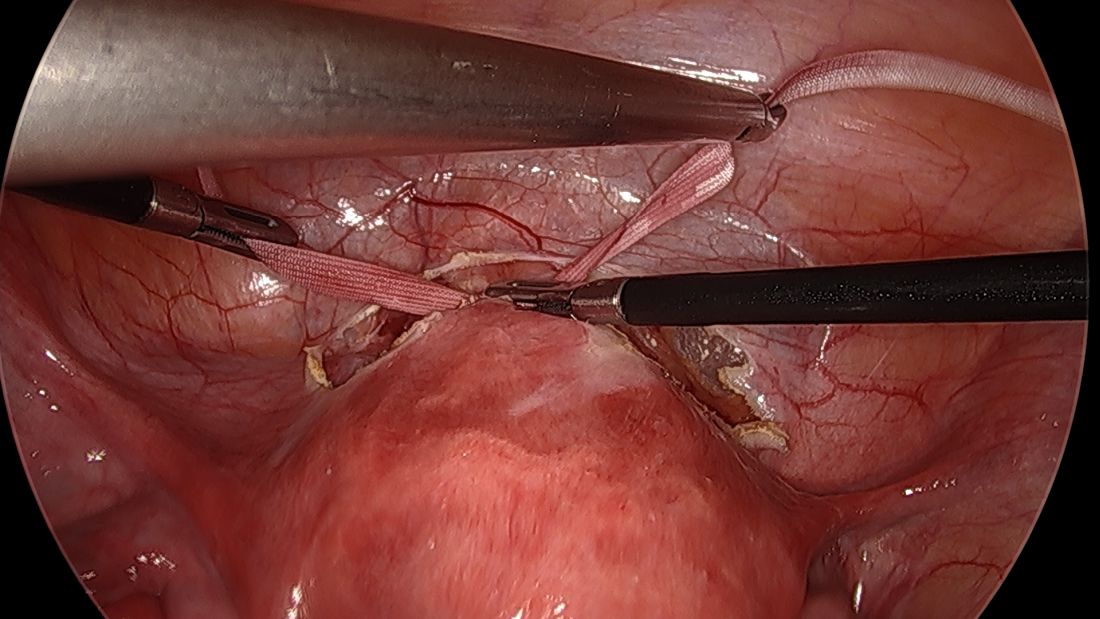
I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.
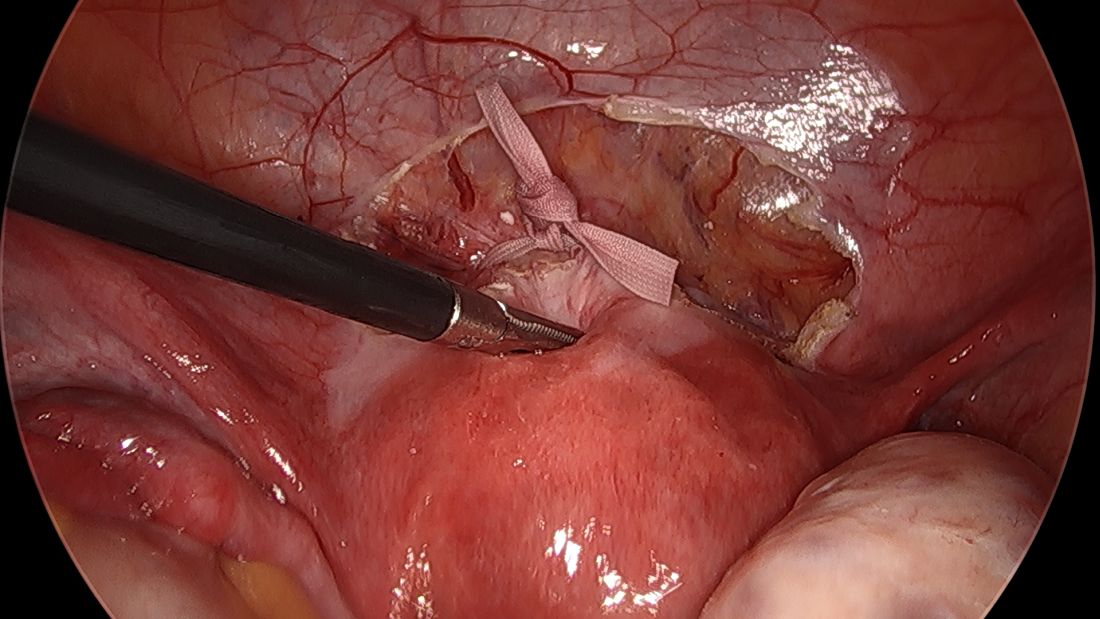
The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.
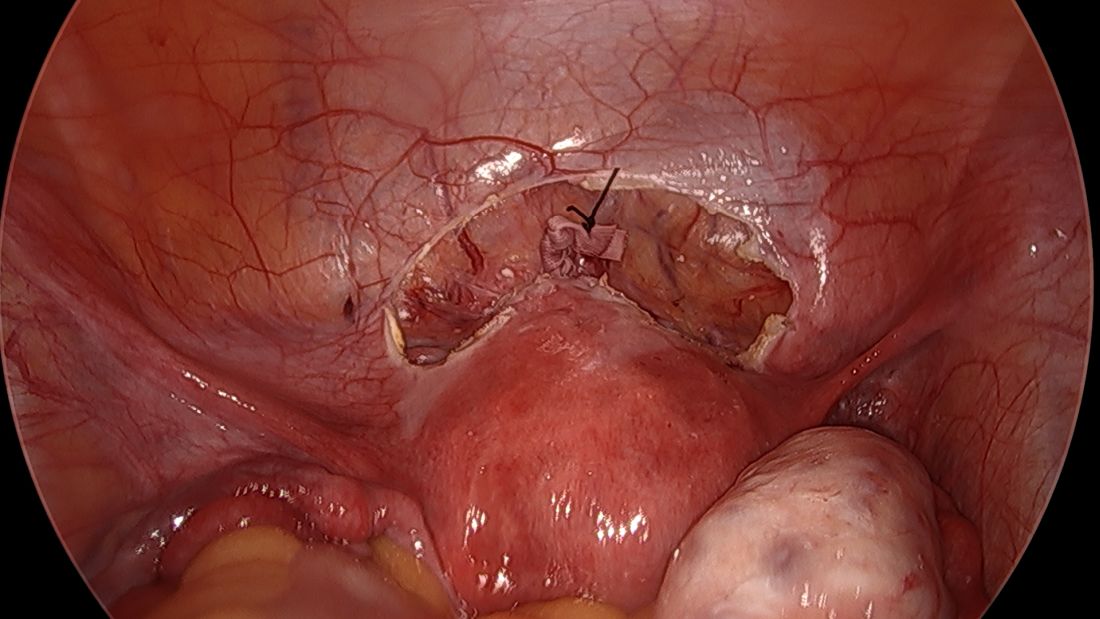
We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.
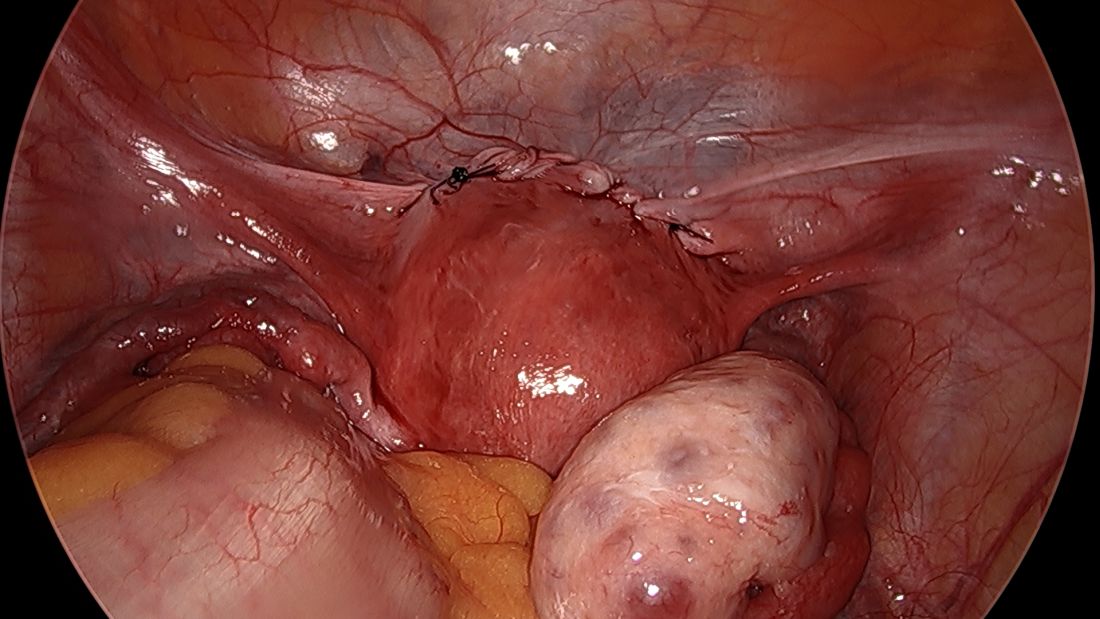
We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.
We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Cervical insufficiency is an important cause of preterm birth and complicates up to 1% of pregnancies. It is typically diagnosed as painless cervical dilation without contractions, often in the second trimester at around 16-18 weeks, but the clinical presentation can be variable. In some cases, a rescue cerclage can be placed to prevent second trimester loss or preterm birth.
A recent landmark randomized controlled trial of abdominal vs. vaginal cerclage – the MAVRIC trial (Multicentre Abdominal vs. Vaginal Randomized Intervention of Cerclage)1 published in 2020 – has offered significant validation for the belief that an abdominal approach is the preferred approach for patients with cervical insufficiency and a prior failed vaginal cerclage.
Obstetricians traditionally have had a high threshold for placement of an abdominal cerclage given the need for cesarean delivery and the morbidity of an open procedure. Laparoscopic abdominal cerclage has lowered this threshold and is increasingly the preferred method for cerclage placement. Reported complication rates are generally lower than for open abdominal cerclage, and neonatal survival rates are similar or improved.
In our experience, the move toward laparoscopic abdominal cerclage is largely a patient-driven shift. Since 2007, at Brigham and Women’s Hospital in Boston, we have performed over 150 laparoscopic abdominal cerclage placements. The majority of patients had at least one prior second-trimester loss (many of them had multiple losses), with many having also failed a transvaginal cerclage.
In an analysis of 137 of these cases published recently in Fertility and Sterility, the neonatal survival rate was 93.8% in the 80 pregnancies that followed and extended beyond the first trimester, and the mean gestational age at delivery was 36.9 weeks.2 (First trimester losses are typically excluded from the denominator because they are unlikely to be the result of cervical insufficiency.)
History and outcomes data
The vaginal cerclage has long been a mainstay of therapy because it is a simple procedure. The McDonald technique, described in the 1950s, uses a simple purse string suture at the cervico-vaginal juncture, and the Shirodkar approach, also described in the 1950s, involves placing the cerclage higher on the cervix, as close to the internal os as possible. The Shirodkar technique is more complex, requiring more dissection, and is used less often than the McDonald approach.
The abdominal cerclage, first reported in 1965,3 is placed higher on the cervix, right near the juncture of the lower uterine segment and the cervix, and has generally been thought to provide optimal integrity. It is this point of placement – right at the juncture where membranes begin protruding into the cervix as it shortens and softens – that offers the strongest defense against cervical insufficiency.
The laparoscopic abdominal approach has been gaining popularity since it was first reported in 1998.4 Its traditional indication has been after a prior failed vaginal cerclage or when the cervix is too short to place a vaginal cerclage – as a result of a congenital anomaly or cervical conization, for instance.
Some of my patients have had one pregnancy loss in which cervical insufficiency was suspected and have sought laparoscopic abdominal cerclage without attempting a vaginal cerclage. Data to support this scenario are unavailable, but given the psychological trauma of pregnancy loss and the minimally invasive and low-risk nature of laparoscopic abdominal cerclage, I have been inclined to agree to preventive laparoscopic abdominal procedures without a trial of a vaginal cerclage. I believe this is a reasonable option.
The recently published MAVRIC trial included only abdominal cerclages performed using an open approach, but it provides good data for the scenario in which a vaginal cerclage has failed.
The rates of preterm birth at less than 32 weeks were significantly lower with abdominal cerclage than with low vaginal cerclage (McDonald technique) or high vaginal cerclage (Shirodkar technique) (8% vs. 33%, and 8% vs. 38%). No neonatal deaths occurred.
The analysis covered 111 women who conceived and had known pregnancy outcomes, out of 139 who were recruited and randomized. Cerclage placement occurred either between 10 and 16 weeks of gestation for vaginal cerclages and at 14 weeks for abdominal cerclages or before conception for those assigned to receive an abdominal or high vaginal cerclage.
Reviews of the literature done by our group1 and others have found equivalent outcomes between abdominal cerclages placed through laparotomy and through laparoscopy. The largest systematic review analyzed 31 studies involving 1,844 patients and found that neonatal survival rates were significantly greater in the laparoscopic group (97% vs. 90%), as were rates of deliveries after 34 weeks of gestation (83% vs. 76%).5
The better outcomes in the laparoscopic group may at least partly reflect improved laparoscopic surgeon techniques and improvements in neonatal care over time. At the minimum, we can conclude that neonatal outcomes are at least equivalent when an abdominal cerclage is placed through laparotomy or with a minimally invasive approach.
Our technique
Laparoscopic cerclages are much more easily placed – and with less risk of surgical complications or blood loss – in patients who are not pregnant. Postconception cerclage placement also carries a unique, small risk of fetal loss (estimated to occur in 1.2% of laparoscopic cases and 3% of open cases). 1 We therefore prefer to perform the procedure before pregnancy, though we do place abdominal cerclages in early pregnancy as well. (Approximately 10% of the 137 patients in our analysis were pregnant at the time of cerclage placement. 1 )
The procedure, described here for the nonpregnant patient, typically requires 3-4 ports. My preference is to use a 10-mm scope at the umbilicus, two 5-mm ipsilateral ports, and an additional 5-mm port for my assistant. We generally use a uterine manipulator to help with dissection and facilitate the correct angulation of the suture needle.
We start by opening the vesicouterine peritoneum to dissect the uterine arteries anteriorly and to move the bladder slightly caudad. It is not a significant dissection.
For suturing, we use 5-mm Mersilene polyester tape with blunt-tip needles – the same tape that is commonly used for vaginal cerclages. The needles (which probably are unnecessarily long for laparoscopic cerclages) are straightened out prior to insertion with robust needle holders.

The posterior broad ligament is not opened prior to insertion of the needle, as opening the broad ligament risks possible vessel injury and adds complexity.

Direct insertion of the needle simplifies the procedure and has not led to any complications thus far.
We prefer to insert the suture posteriorly at the level of the internal os just above the insertion of the uterosacral ligaments. It is helpful to view the uterus and cervix as an hourglass, with the level of the internal os is at the narrowest point of the hourglass.
The suture is passed carefully between the uterine vessels and the cervical stroma. The uterine artery should be lateral to placement of the needle, and the uterosacral ligament should be below. The surgeon should see a pulsation of the uterine artery. The use of blunt needles is advantageous because, especially when newer to the procedure, the surgeon can place the needle in slightly more medial than may be deemed necessary so as to avert the uterine vessels, then adjust placement slightly more laterally if resistance is met.
Suture placement should follow a fairly low-impact path. Encountering too much resistance with the needle signals passage into the cervix and necessitates redirection of the needle with a slightly more lateral placement. Twisting the uterus with the uterine manipulator can be helpful throughout this process.
Once the needles are passed through, they are cut off the Mersilene tape and removed. For suturing, it’s important that the first and second knots are tied down snuggly and flat.

I usually ask my assistant to hold down the first knot so that it doesn’t unravel while I tie the second knot. I usually tie 6 square knots with the tape.

The edges of the tape are then trimmed, and with a 2.0 silk suture, the ends are secured to the lower uterine segment to prevent a theoretical risk of erosion into the bladder.

We then close the overlying vesicouterine peritoneum with 2-0 Monocryl suture, tying it intracorporally. Closing the peritoneum posteriorly is generally not necessary.

We have not had significant bleeding or severe complications in any of our cases. And while the literature comparing preconception and postconception abdominal cerclage is limited, the risks appear very low especially before pregnancy. Some oozing from the uterine vein can sometimes occur; if this does not resolve once it is tied down, placement of a simple figure of eight suture such as a Monocryl or Vicryl at the posterior insertion of the tape may be necessary to stop the bleeding.
Some surgeons place the abdominal cerclage lateral to the uterine artery, presumably to lessen any risk of vessel injury, but again, our placement medial to the vessels has not led to any significant bleeding. By doing so we are averting a theoretical risk with lateral placement of possibly constricting blood flow to the uterus during pregnancy.
Another technique for suturing that has been described uses a fascial closing device, which, after the needles are removed, passes between the vessels and cervix anteriorly and grasps each end of the suture posteriorly before pulling it through the cervix. My concern with this approach is that entry into the cervix with this device’s sharp needles could cause erosion of the tape into the cervical canal. Piercing of a vessel could also cause bleeding.
Laparoscopic abdominal cerclage can also be placed with robotic assistance, but I don’t believe that the robot offers any benefit for this relatively short, uncomplicated procedure.
A note on patient care
We recommend that patients not become pregnant for 2 months after the laparoscopic abdominal cerclage is placed, and that they receive obstetrical care as high-risk patients. The cerclage can be removed at the time of cesarean delivery if the patient has completed childbearing. Otherwise, if the cerclage appears normal, it can be left in place for future pregnancies.
In the event of a miscarriage, a dilatation and evacuation procedure can be performed with an abdominal cerclage in place, up to 18 weeks of pregnancy. Beyond this point, the patient likely will need to have the cerclage removed laparoscopically to allow vaginal passing of the fetus.
References
1. Shennan A et al. Am J Obstet Gynecol. 2020;222(3):261.E1-261.E9.
2. Clark NV & Einarsson JI. Fertil Steril. 2020;113:717-22.
3. Benson RC & Durfee RB. Obstet Gynecol. 1965;25:145-55.
4. Lesser KB et al. Obstet Gynecol. 1998;91:855-6.
5. Moawad GN et al. J Minim Invasive Gynecol. 2018;25:277-86.
Laparoscopic approach to abdominal cerclage
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Preterm birth remains a significant cause of infant morbidity and mortality. A well-established cause of preterm birth is cervical insufficiency, which occurs in approximately 1% of pregnancies and up to 8% of recurrent miscarriages and midtrimester pregnancy loss. A cerclage, a purse-string suture around the cervix, is placed to treat cervical insufficiency and, thus, prevent second-trimester loss and preterm birth. While, traditionally, placement of the cerclage was performed via a vaginal route, over the past 50 years, abdominal cerclage has been utilized in cases in which a vaginal cerclage has failed or the cervix is extremely short. The advantage of the abdominal approach is the ability to place the suture at the level of the internal os. Moreover, there is no potential risk of ascending infection and resultant preterm labor or premature rupture of membranes secondary to a foreign body in the vagina, as in the case of vaginal cerclage. There has been a reluctance to perform abdominal cerclage as a first-time treatment secondary to the need for cesarean section, risk of hemorrhage at the uterine vessels, and in the past, the need for a laparotomy.
With the introduction of a laparoscopic or robot-assisted approach to abdominal cerclage in preterm birth prevention, there has been an upsurge in the popularity of abdominal cerclage as the first-line surgical procedure, especially after a failed vaginal cerclage. In 2018, Moawad et al., in a systematic review of laparoscopic abdominal cerclage, noted slight improvement in neonatal outcomes with laparoscopy vs. laparotomy.
For this edition of the Master Class in gynecologic surgery, I have enlisted the assistance of Jon I. Einarsson, MD, PhD, MPH, who is chief of the division of minimally invasive gynecology at Brigham and Women’s Hospital and professor of obstetrics/gynecology at Harvard Medical School, Boston. Dr. Einarsson is a past president of the American Association of Gynecologic Laparoscopists. He is a very well-known, published clinical researcher and surgical innovator. Dr. Einarsson is the founder of Freyja Healthcare, a privately held medical device company advancing women’s health through innovation.
It is a pleasure and honor to welcome my friend and colleague, Dr. Jon I. Einarsson, to this edition of the Master Class in gynecologic surgery.
Dr. Miller is professor of obstetrics and gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
How advances in genomics have informed obstetrics practice
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Genetic screening and diagnosis: Key advancements and the role of genetic counseling
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)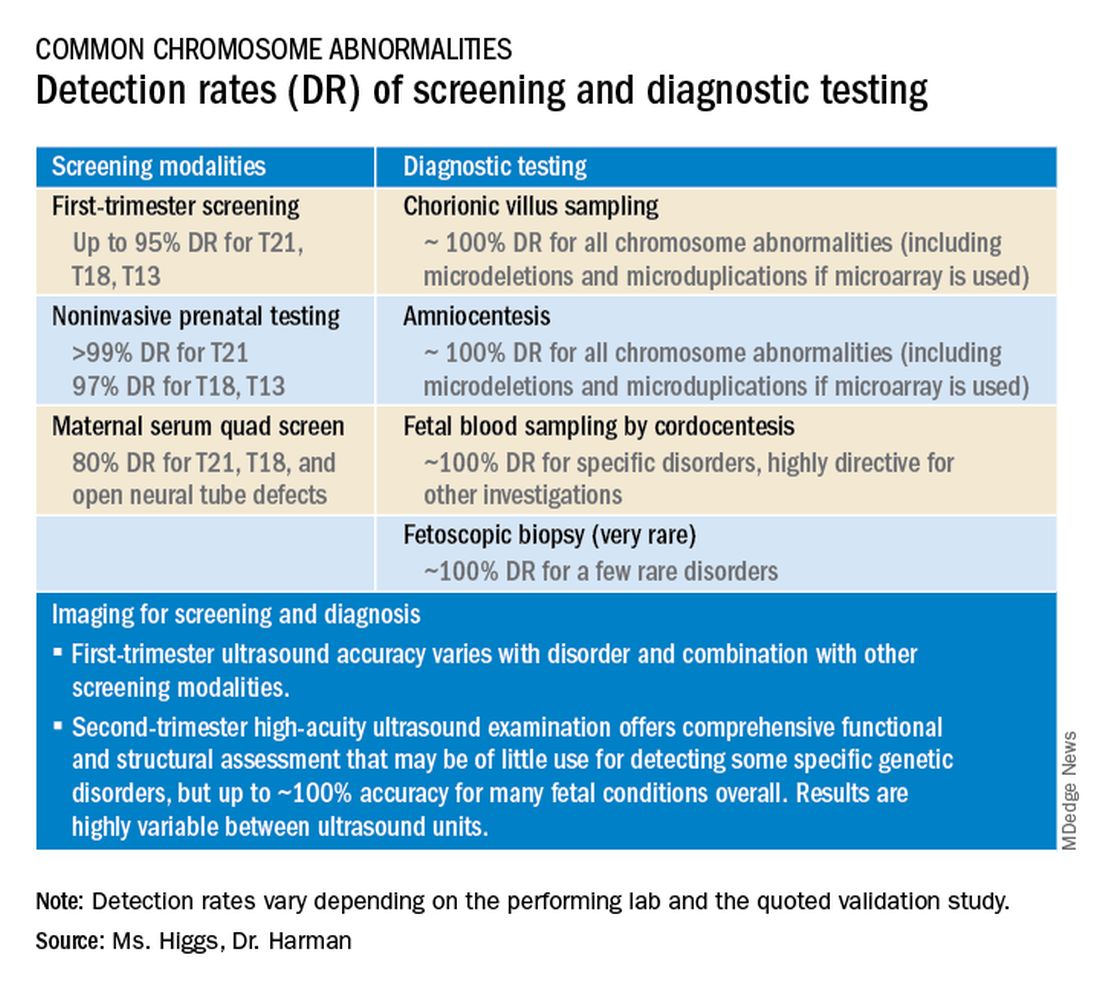
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.
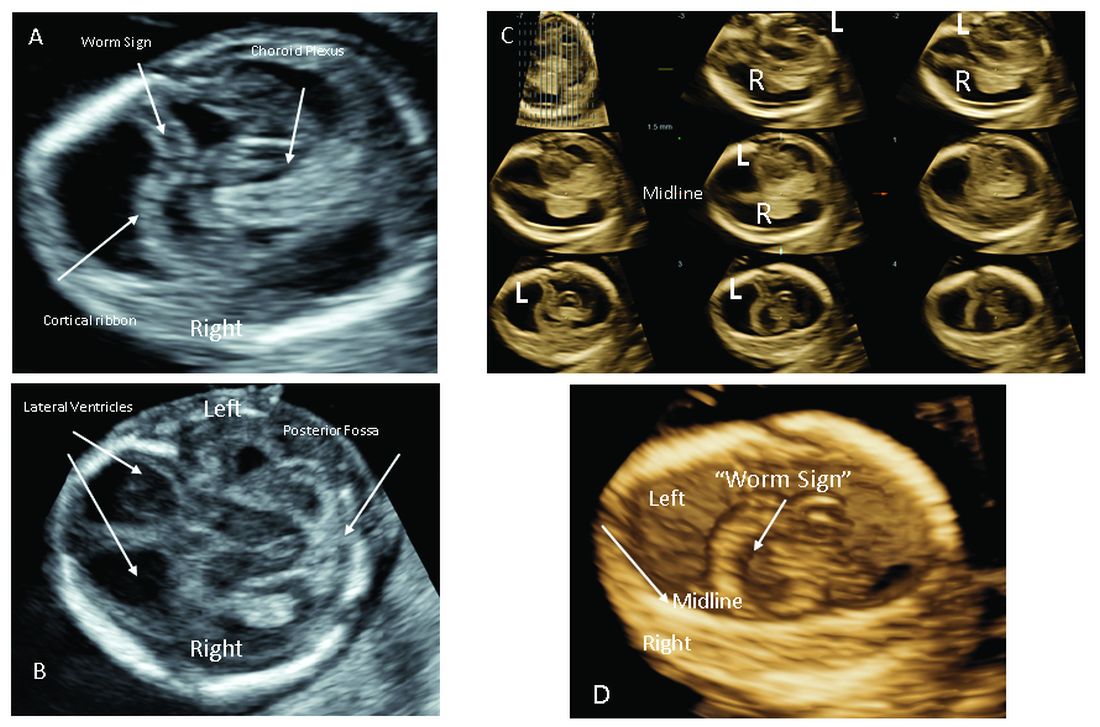
Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
obnews@mdedge.com
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
obnews@mdedge.com
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
obnews@mdedge.com
The current and future state of uterus transplantation
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.