User login
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
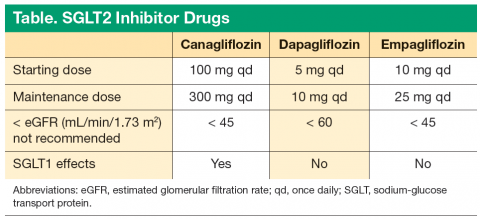
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
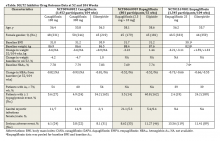
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Over the past 2 decades, the treatment of type 2 diabetes mellitus (T2DM) has been an evolving science. With therapeutic advances, the prevalence of catastrophic complications such as amputations, renal failure requiring dialysis, and blindness due to retinopathy have significantly declined. Developed drugs have successfully met treatment goals; however, they are often associated with a higher risk of hypoglycemia and weight gain. Now that better glucose control is possible, the science of diabetes care continues to evolve. Newly developed drugs should control glucose without significant hypoglycemia and also promote weight reduction. The sodium-glucose transport protein 2 (SGLT2) inhibitor drug class has these characteristics, and the novel mechanism of action complements older medications used to treat T2DM.
Phlorizin is a plant-based compound originally discovered in 1935 when it was derived from the bark of apple trees.1 It is a naturally occurring botanical glucoside and is fairly nonselective between SGLT1 and SGLT2. Due to its poor bioavailability and its degradation in the gastrointestinal tract, it was not an ideal drug candidate in humans.
Canagliflozin
Canagliflozin is a SGLT2 inhibitor and a low-potency SGLT1 inhibitor. It was the first SGLT2 inhibitor approved by the FDA (March 2013) to be used with diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 100 mg once daily for patients who have an estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 and can be increased to 300 mg once daily. It is also available in a fixed-dose combination with metformin. Canagliflozin SGLT-2 inhibition leads to increased glycosuria and osmotic diuresis that lowers plasma glucose concentrations. Lower blood pressure (BP) is likely an effect of the osmotic diuresis. Increased urinary excretion of glucose also leads to a loss of calories and weight loss. It was studied alone and in combination with metformin, sulfonylurea, pioglitazone, and insulin therapy.
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with T2DM. Peak plasma concentrations (Cmax) and area under the cover (AUC) of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, time to Cmax (Tmax) of canagliflozin occurs within 1 to 2 hours postdose. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Glucuronidation is the major metabolic pathway. There is balanced renal and biliary excretion of metabolites, and there are no active metabolites.
Following oral doses of canagliflozin in patients with T2DM, dose-dependent decreases were seen in the renal threshold for glucose (RTG). From a starting value of about 240 mg/dL, the 300-mg dose suppressed the mean (RTG) to about 70 to 90 mg/dL in T2DM in phase 1 studies. The reduction in RTG led to increase in urinary excretion of glucose of about 100 g/d.
In addition to renal SGLT2 inhibition leading to increased urinary glucose excretion (UGE), canagliflozin has been shown to lower postprandial glucose excursion (PPGE) and insulin concentrations by delaying intestinal glucose absorption.2 A study was done in 20 healthy subjects who received either placebo or canagliflozin 300 mg 20 minutes before a 600 kcal mixed-meal tolerance test. Compared with placebo, canagliflozin reduced PPGE and insulin excursions (0-2 h) AUC by 35% and 43%, respectively (P < .001 for both). This may present a difference between canagliflozin and the other SGLT2 inhibitors.
Because of the potential differences due to canagliflozin’s inhibition of intestinal SGLT1, the pharmacodynamic differences between canagliflozin and dapagliflozin were studied.3 The randomized, double-blind, crossover study consisted of 54 subjects. The subjects received the maximum approved doses of canagliflozin 300 mg or dapagliflozin 10 mg a day. Each group was treated with the study drug for 2 days, and then a 600 kcal mixed-meal tolerance test was performed. The results of the PPGE 0- to 2-hour AUC analysis showed 3.66 mmol*h/L with canagliflozin 300 mg and 4.08 mmol*h/L with dapagliflozin 10 mg. There was a difference of 0.42 (P = .0122), which was a 10.3% reduction in AUC PPGE by canagliflozin compared with dapagliflozin.
Canagliflozin has been studied in patients with T2DM and stage 3 nephropathy. Data were pooled from 4 randomized, placebo-controlled, phase 3 studies in which subjects had baseline eGFR > 30 to < 60 mL/min/1.73 m2.4 In the setting of decreased eGFR associated with stage 3 chronic kidney disease, subjects treated with canagliflozin 100 mg and canagliflozin 300 mg had placebo-subtracted reductions in hemoglobin A1c (A1c) of -0.38% and -0.47%, respectively, and placebosubtracted reduction in weight of -1.6% and -1.9%, respectively. Decreases in eGFR were seen at week 6 but trended toward baseline over time with a mean change in eGFR of 0.7, -1.7, -2.2 mL/min/1.73 m2 for placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Clinical Efficacy Trials
Canagliflozin was studied as add-on therapy to metformin and compared with glimepiride (a sulfonylurea).5 The randomized, double-blind study included 1,450 subjects for a core study period of 52 weeks followed by a 52-week extension. Eligible subjects were aged > 18 and < 80 years, A1c > 7% and < 9.5%, and were receiving metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. The study groups were canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, and baseline A1c measurements were 7.78%, 7.79%, and 7.83%, respectively. The glimepiride was titrated up if > 50% of fasting blood glucose measurements were > 108 mg/dL with no hypoglycemic events in the previous 2 weeks.
Over 104 weeks, canagliflozin 100 mg and 300 mg and glimepiride reduced A1c from mean baseline values by -0.65%, -0.74%, and -0.55%, respectively, and the proportions of patients achieving A1c < 7% at week 104 was 42.5%, 50.2%, and 43.9%, respectively. Weight fell over 104 weeks with canagliflozin 100 mg (-4.1%, -3.6 kg) and canagliflozin 300 mg (-4.2%, -3.6 kg). In contrast, glimepiride showed weight increase (0.9%, 0.8 kg). Documented hypoglycemia episodes were lower in canagliflozin 100 mg and 300 mg than with glimepiride (6.8%, 8.2%, and 40.9%, respectively).
A study was undertaken to compare canagliflozin with the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin in patients with T2DM on background therapy of metformin.6 This randomized, double-blind trial studied subjects aged > 18 and < 80 years with inadequate glucose control A1c > 7% and < 10.5%. Subjects received metformin > 2,000 mg/d or > 1,500 mg/d if unable to tolerate a higher dose. At week 52, canagliflozin showed noninferiority to sitagliptin, with both drugs lowering A1c by 0.73%. Canagliflozin 300 mg showed superiority to sitagliptin with -0.88% change in A1c. Both canagliflozin 100 mg and 300 mg were superior to sitagliptin 100 mg in weight reduction: -3.8%, -4.2%, and -1.3%, respectively. Genital mycotic infections were higher in the canagliflozin groups. Rates for mycotic infections for sitagliptin 100 mg, canagliflozin 100 mg, and canagliflozin 300 mg were 1.2%, 5.2%, and 2.4% in men, respectively, and 2.6%, 11.3%, and 9.9% in women, respectively.
Canagliflozin was also studied in combination with insulin to determine efficacy and safety in this setting.7 Subjects were randomized to receive placebo, canagliflozin 100 mg, or canagliflozin 300 mg. Subjects had a mean baseline A1c of 8.3%. The median daily insulin dose was 60 IU, and most individuals were using basal/bolus regimens. The primary endpoint was 18 weeks of therapy, and A1c was lowered 0.62% (P < .001) with canagliflozin 100 mg and 0.73% with canagliflozin 300 mg compared with placebo. Weight decreased 1.9% (P < .001) with canagliflozin 100 mg and 2.4% (P < .001) with canagliflozin 300 mg compared with placebo.
Adverse Effects and Precautions
Canagliflozin adverse effects (AEs) were generally low. In the aforementioned 104-week study comparing canagliflozin 100 mg, canagliflozin 300 mg, and glimepiride, AEs leading to discontinuation were low at 6.2%, 9.5%, and 7.3%, respectively. Serious AEs were lower in the canagliflozin 100 mg and 300 mg groups compared with glimepiride at 9.7%, 9.7%, 14.3%, respectively.5
Limitations for use of canagliflozin are T1DM and diabetic ketoacidosis (DKA). In mild renal impairment, there is no dose adjustment in patients with eGFR > 60 mL/min/1.73 m2. In moderate renal impairment (eGFR 45-60 mL/min/1.73 m2), dose is limited to 100 mg once daily. It is recommended not to initiate canagliflozin if eGFR is < 45 mL/min/1.73 m2. Canagliflozin is contraindicated if eGFR is < 30 mL/min/1.73 m2.8
Dapagliflozin
Dapagliflozin is a highly selective SGLT2 inhibitor. It is a 1,400-fold greater inhibitor of SGLT2 vs SGLT1 and was approved in January 2014. It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. The recommended starting dose is 5 mg once daily, taken in the morning, with or without food, and the dosage can be increased to 10 mg once daily in patients who require additional glycemic control. Dapagliflozin should not be initiated if eGFR is < 60 mL/min/1.73 m2, and it should be discontinued if eGFR is persistently < 60 mL/min/1.73 m2.
By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose, thereby increasing UGE. Increased glucose secretion also leads to weight reduction. Dapagliflozin has been studied alone and in combination with glipizide, glimepiride, pioglitazone, and a DDP-4 inhibitor and as an add-on to insulin with and without other oral antidiabetic drugs. It is also available in a fixed-dose combination with metformin.
Following oral administration of dapagliflozin, the Tmax is usually attained within 2 hours under a fasting state. The Cmax and AUC values increase the dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10-mg dose is 78%. The mean plasma t1⁄2 for dapagliflozin is about 12.9 hours following a single oral dose of 10 mg. In patients with normal renal function, the renal glucose excretion was 85 g per day at maximal dose. In humans, 75% of the dose of dapagliflozin is primarily metabolized through the uridine diphosphate glucuronosyltransferase 1A9 pathway. Dapagliflozin and related metabolites are primarily eliminated via the renal pathway.9
Clinical Efficacy Trials
Dapagliflozin was studied as add-on therapy to metformin with glipizide (a sulfonylurea) as the comparator.10 This 52-week double-blind, multicenter, active-controlled, noninferiority trial randomized 801 patients. Eligible subjects were aged > 18 years with A1c > 6.5% and < 10% and were receiving metformin or metformin and 1 other oral antidiabetic drug up to half-maximal dose for at least 8 weeks. During an 18-week titration period, all patients started dapagliflozin 2.5 mg/d and glipizide 5 mg/d. At 21-day intervals, patients were titrated up to the next dosage level if fasting plasma glucose was > 110 mg/dL. Dapagliflozin could be titrated to 5 mg and then 10 mg per protocol. Glipizide could be titrated to 10 mg or 20 mg per protocol.
The A1c change with dapagliflozin was noninferior to glipizide at week 52. The A1c adjusted mean change from baseline was -0.52 for both dapagliflozin and glipizide. The secondary endpoint was weight change. The glipizide group had a +1.44 kg weight gain, whereas the dapagliflozin group had a -3.22 kg weight loss. The number of patients with ≥ 1 episode of hypoglycemia, either symptomatic or with no symptoms, with blood glucose ≤ 63 mg/dL was assessed. The dapagliflozin group had a 3.5% rate compared with the glipizide group, which had a 40.8% rate of patients with > 1 hypoglycemic episode.
A study examined the efficacy and safety of dapagliflozin in combination with and also vs a DPP-4 inhibitor with metformin background therapy. The study looked at add-ons of saxagliptin plus dapagliflozin vs saxagliptin or dapagliflozin added alone.11 This was a randomized, double-blind, 24-week study with patients aged > 18 years with inadequate glucose control A1c > 8.0% and < 12.0%. Patients had to be on a stable dose of metformin > 1,500 mg/d for at least 8 weeks. Patients were randomized 1:1:1 to receive either saxagliptin 5 mg/d and dapagliflozin 10 mg/d plus metformin, saxagliptin 5 mg/d and placebo plus metformin, or dapagliflozin 10 mg/d and placebo plus metformin.
The patients had a mean age of 54 years and a mean duration of T2DM of 7.6 years. The mean baseline A1c was 8.94%. The addition of saxagliptin plus dapagliflozin to metformin resulted in significantly greater A1c reduction compared with saxagliptin plus metformin or dapagliflozin plus metformin from baseline A1c levels: -1.47%, -0.88%, -1.2%, respectively.11
Dapagliflozin was also studied in combination with insulin to determine the safety and efficacy in this setting.12 This double-blind, placebo-controlled, parallel-group trial had an initial study period of 24 weeks followed by an extension period totaling 104 weeks. At 48 weeks, patients on dapagliflozin 5 mg were switched to 10 mg. Outcomes over 104 weeks were changed from baseline A1c, insulin dose, and body weight. Up-titration of insulin was permitted if at least 3 self-monitored blood glucose readings from the 7 days prior to the study visit were > 240 mg/dL up to week 12; > 220 mg/dL between weeks 12 and 24; > 178 mg/dL or if A1c was > 8% between weeks 24 and 48. Between weeks 52 and 65, insulin titrated up was allowed if A1c was > 7.5% and between weeks 78 and 104 if A1c was > 7%. Insulin could be titrated down if ≥ 2 self-monitored blood glucose readings were < 68 mg/dL.
The study group had a T2DM diagnosis for 13.6 years and a mean duration of insulin therapy for about 6 years. The mean daily insulin dose was 77.1 IU. The mean baseline A1c was 8.5%. At 104 weeks, the differences from placebo in A1c adjusted mean change from baseline were -4.0% (P = .0002) and -0.4% (P = .0007) in the dapagliflozin 5 mg (switched to 10 mg at week 48) and 10 mg groups, respectively. Insulin requirements increased progressively in the placebo group +18.3 IU/d at 104 weeks. Insulin requirements stayed stable over 104 weeks in the dapagliflozin groups. Body weight increased in the placebo group, whereas it decreased in the dapagliflozin groups. At 104 weeks, the weight changes from baseline in placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg were -0.99 kg, -1.03 kg (P < .001), and -1.5 kg (P < .0001), respectively. The frequency of ≥ 1 minor or major episodes of hypoglycemia was fairly balanced across placebo, dapagliflozin 5 mg/10 mg, and dapagliflozin 10 mg at 61.9%, 61.3%, and 60.7%, respectively.
Adverse Effects and Precautions
Dapagliflozin was generally well tolerated. In the trial of dapagliflozin compared with glipizide in the setting of metformin background therapy, AEs led to discontinuation rates of 9.1% vs 5.9%, respectively. Serious AEs were lower in the dapagliflozin group compared with glipizide at 8.6% vs 11.3%, respectively.10
Limitations for use of dapagliflozin are the treatment of T1DM or the treatment of DKA. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild renal impairment (eGFR of ≥ 60 mL/ min/1.73 m2), and dapagliflozin should be discontinued when eGFR is persistently < 60 mL/min/1.73 m2. In the clinical trials, there was an imbalance in the number of bladder cancer reported with dapagliflozin compared with placebo. Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10 of 6,045 patients (0.17%) treated with dapagliflozin and 1 of 3,512 patients (0.03%) treated with placebo or a comparator.9 There were too few cases to determine whether the emergence of these events is related to dapagliflozin. Product labeling states dapagliflozin should not be used in patients with active bladder cancer and should be used with caution in those with a prior history of bladder cancer.
Empaglifilozin
Empagliflozin is the most recently FDA-approved SGLT2 inhibitor (August 2014) for improved glycemic control in T2DM. It has the highest SGLT2 selectivity: > 2,500-fold selectivity for SGLT2 over SGLT1.13 Empagliflozin regulates blood glucose levels by increased UGE, independent of endogenous insulin secretion. It is associated with modest reductions in body weight, visceral adiposity, and systolic BP.
Empagliflozin is available in 10-mg and 25-mg tablets, with a recommended initial dose of 10 mg daily.13 Dosing adjustments are not required for geriatric patients or for those patients with hepatic impairment.14 The use of empagliflozin is contraindicated in patients with eGFR < 45 mL/min/1.73 m2 or for those whose eGFR declines to < 45 mL/min/1.73 m2 during therapy.15,16 Empagliflozin has a pregnancy risk category C. Drug transference during lactation is unknown; therefore, empagliflozin during breast-feeding is not recommended. It is also available in a fixed-dose combination with metformin.
Empagliflozin has been studied alone and in combination therapy with other oral antidiabetic drugs as well as insulin therapy. Metformin, pioglitazone, sitagliptin, and linagliptin have been studied in combination with empagliflozin with sustained glycemic improvement without significantly increased risk of hypoglycemia.17-21 Empagliflozin/linagliptin combination was recently approved after phase 3 trials demonstrated 62% of patients achieved an A1c value < 7% on the 25/5-mg dose at 24 weeks.21 Empagliflozin, coadministered with multiple daily injections of insulin (MDI), has been shown to safely improve glycemic control and reduce total daily insulin requirements without an increased risk of hypoglycemia.22 Currently, it is not approved for use in patients with T1DM, but phase 3 trials are ongoing.
Pharmacokinetics of empagliflozin among healthy volunteers paralleled those of people with T2DM with rapid absorption. The plasma glucose lowering effect of empagliflozin was evident after the first dose and became more pronounced with treatment duration. The AUC and Cmax were dose-proportional over a range of empagliflozin doses in a single rising dose study, with maximum UGE of 90.8 g in healthy volunteers reached at the 400-mg dose.23 The Tmax was 1.5 to 2.1 hours after dosing, comparable to dapagliflozin.24 Steady state with once-daily dosing is reached by day 5 with t1/2 range of 10 to 19 hours.24-27 Plasma levels of empagliflozin declined in a biphasic pattern, with a rapid distribution phase and a slower elimination phase. Total urine volume did not differ significantly in the empagliflozin group compared with placebo.
Healthy subjects treated with placebo or empagliflozin had comparable plasma glucose concentrations. Patients with T2DM demonstrated a decrease in mean daily glucose of -37.0 mg/dL for the 10-mg dose compared with -13.5 mg/dL for placebo. Doses up to 10 mg were found to inhibit renal tubular reabsorption up to 40%, and higher doses inhibit up to 60% of filtered glucose.22-26 Empagliflozin has been shown to have similar efficacy independent of food.27 The pharmacodynamic response declines with increasing renal impairment in SGLT2 inhibitors. Empagliflozin has been associated with a decline from UGE of 97.6 g in normal renal function to 18.2 g in severe renal impairment.
Phase 1 studies of healthy male volunteers initially elucidated empagliflozin’s therapeutic potential of dosedependent increases in UGE and associated reduction in A1c with daily administration, respectively.24,28 Single rising dose studies further defined the linear pharmacokinetics and excellent tolerability of empagliflozin.23 Heise and colleagues demonstrated in 2 separate studies with multiple oral doses (2.5 mg, 10 mg, 25 mg, and 100 mg) of empagliflozin in people with T2DM similar pharmacokinetics and efficacy in UGE, tolerability, and reduction in plasma glucose.24
Ferrannini and colleagues, in a 12-week phase 2 study, demonstrated a statistically significant reduction in A1c of 0.5% and 0.6% with 10-mg and 25-mg doses, respectively, as well as a universal body weight decline by 2 kg.29
Clinical Efficacy Trials
A phase 3 trial of 1,549 randomized people with T2DM (aged > 18 years with baseline A1c of 7%-10%), comparing empagliflozin and glimepiride as an add-on to metformin, demonstrated noninferiority at 52 weeks and statistically significant superiority for the empagliflozin group at 104 weeks.30 The adjusted mean difference in change from baseline in A1c with empagliflozin vs glimepiride at week 104 was -0.11% (95% confidence interval, -0.19 to -0.02; P = .0153 for superiority). Despite 39% of both groups achieving a A1c < 7% at week 52, the empagliflozin group had significantly less hypoglycemia (2% vs 24%). In the body composition substudy, empagliflozin demonstrated a significant reduction of 3 kg compared with an increase of slightly over 1 kg in the glimepiride group at week 104. The majority of the empagliflozin-associated weight loss was found to be a reduction in fat mass.
An international phase 2B randomized, controlled, open-label extension study of 659 people with T2DM (aged > 18 and < 79 years with a body mass index (BMI) < 40 kg/m2 and baseline A1c of 7%-10%) described the long-term safety and efficacy of empagliflozin monotherapy in combination with metformin as compared with sitagliptin monotherapy in combination with metformin. At week 90, the changes from baseline A1c were -0.34%/ -0.47% in the empagliflozin 10 mg/25 mg monotherapy group, -0.34%/0.63% in the empagliflozin 10 mg/25 mg/ metformin combination, -0.56% with metformin monotherapy, and -0.40% with sitagliptin/metformin combination.31 These data provided evidence that empagliflozin had sustained weight loss effects (-2.2 kg to -4.0 kg).
Empagliflozin monotherapy was studied with sitagliptin as an active comparator in a phase 3 trial of 899 people with T2DM (aged > 18 years with baseline A1c of 7%-10%), with the primary endpoint of change in baseline in A1c at week 24. Both the 10-mg and 25-mg dose sof empagliflozin were associated with greater reductions in A1c from baseline (-0.66%, -0.77%) at week 24 than that of sitagliptin (-1.04%). Additionally, both doses of empagliflozin were associated with greater reductions in bodyweight (-2.26 kg and -2.48 kg) as compared with sitagliptin 100 mg (+0.18 kg).20
Empagliflozin’s efficacy and safety in combination with MDI insulin as well as add-on to basal insulin was tested in 2 separate studies.23,32 People with T2DM (mean age 58.8 years, A1c 8.2%) on basal insulin were randomized to empagliflozin (10 mg or 25 mg) or placebo for 78 weeks with a constant basal insulin dose for the first 18 weeks and titration allowed thereafter. Empagliflozin was found to significantly reduce A1c at both 18 and 78 weeks (-0.48% and -0.64% for 10-mg/25-mg doses, respectively, vs -0.02% for placebo) and insulin dose at week 78 vs placebo (-8.8 IU on empagliflozin 10 mg and -11.2 IU on empagliflozin 25 mg).32 Similar rates of hypoglycemia were reported (36.1% of patients on empagliflozin and 35.3% on placebo)
Furthermore, empagliflozin was studied in obese people with uncontrolled, insulin-dependent T2DM (A1c 8.3%, BMI 34.8 kg/m2) on MDI insulin (average 92 IU/d) over a 52-week study.22 Patients were randomized to once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo. The study demonstrated improved glycemic control (A1c reduction of -1.27% and -1.18% on empagliflozin 10 mg and 25 mg doses, respectively, compared with -0.81% on placebo) with lower insulin doses (-9 to -11 IU/d) and weight loss (-1.95 kg and -2.04 kg on empagliflozin 10-mg and 25-mg doses, respectively, compared with 0.44 kg on placebo.) There was no increased risk of hypoglycemia noted.
Adverse Effects and Precautions
Empagliflozin is generally well tolerated with low occurrence of AEs. Adverse effects reported in the pooled empagliflozin phase 3 studies were mild to moderate. Serious AEs reported were higher in the placebo group as compared with those of the empagliflozin subjects but did not result in study discontinuation.33
Drug-drug studies of empagliflozin co-administered with other commonly prescribed medication in T2DM showed very limited, if any, interaction. Empagliflozin had no effect on the pharmacokinetics of warfarin or on its anticoagulant activity; therefore, these 2 drugs were deemed safe to be co-administered. No AEs were reported when combining empagliflozin with the loop diuretic hydrochlorothiazide.33 Due to the mode of action of SGLT2 inhibitors, osmotic diuresis may result in a modest reduction in BP.34
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA.35 The FDA Adverse Event Reporting System identified 20 cases of DKA in patients treated with SGLT2 inhibitors from March 2013 to June 6, 2014. Diabetic ketoacidosis is typically accompanied by levels of ketone bodies > 3,000 μmol/L and develops almost exclusively in states of absolute insulin deficiency. The highest level of ketone bodies observed in patients receiving 25 mg of empagliflozin was 1,449 μmol/L compared with a mean of 1,300 μmol/L in a nondiabetic overnight fast.36 Therefore, it is unlikely that the modest empagliflozin-induced ketosis would increase the risk of developing DKA in the absence of absolute insulin deficiency or extreme ketogenic diet.37 Physiologic explanation at the present time is not clear.
Clinical Application
The SGLT2 inhibitors are the latest of 14 classes of drugs approved to treat T2DM. This class offers many beneficial characteristics besides blood sugar lowering. The drugs lower systolic BP, induce weight loss through diuresis of glucose (calories), and carry low risks for hypoglycemia. The American Association of Clinical Endocrinologists recently published their 2015 diabetes management algorithm.38 In this algorithm, they recognized metformin as first-line therapy along with diet and exercise intensification for the treatment of T2DM.
The glucagon-like peptide-1 (GLP-1) analogs and SGLT2 inhibitors are recognized as plausible second-line drugs. They go together well with metformin, providing powerful additional A1c-lowering effects while inducing weight loss without hypoglycemia. Because all GLP-1 analogs are currently available only in injectable forms, the SGLT2 inhibitors offer the additional advantage of being available in pill forms.
Conclusions
All 3 of the current SGLT2 inhibitors are effective tools for treating T2DM. The 3 drugs share many similar traits, and efficacy is generally similar. Empagliflozin has the highest SGLT2 selectivity, > 2,500-fold selectivity for SGLT2 over SGLT1. However, canagliflozin has mild SGLT1 activity, which may offer additional benefits with regards to attenuating PPGE excursions by delaying intestinal glucose absorption (Table).
The SGLT2 inhibitor class had been shown to be effective when used as monotherapy as well as in combination with other oral antidiabetic medications. All 3 SGLT2 inhibitors have also been shown to be effective in combination with insulin and have similar efficacy in these clinical settings (eTable).
All 3 SGLT2 inhibitors are generally well tolerated. There is less hypoglycemia compared with sulfonylureas. However, when the SGLT2 drugs are combined with drugs that can cause hypoglycemia, such as sulfonylureas and insulin, patients must be monitored for hypoglycemia, and titrating down the sulfonylurea or insulin may be necessary. Genital mycotic infections and urinary tract infections in men and woman are common AEs with SGLT2 drugs. Patients must be advised of these possible AEs, and treatment should be prompt if these AEs occur. Because of the mild osmotic diuresis, patients should be reminded to keep well hydrated. SGLT2s have a very mild effect on increasing low-density lipoprotein cholesterol (LDL-C), so care should be taken to ensure that patients’ LDL-C stays at goal.
The FDA recently released a warning that SGLT2 inhibitors may be associated with a higher risk of developing DKA. Although these reports are rare, clinicians should be vigilant. The FDA has suggested that patients should pay close attention for any signs of DKA and seek medical attention immediately if they experience difficulty breathing, nausea, vomiting, abdominal pain, confusion, and unusual fatigue or sleepiness.35
There are subtle differences in the eGFR thresholds for the use of the 3 drugs. It should be kept in mind that with all 3 drugs, the efficacy decreases as the eGFR decreases. It is recommended not to initiate canagliflozin if the patient’s eGFR is < 45 mL/min/1.73 m2. Dapagliflozin should not be initiated in patients with an eGFR < 60 mL/min/1.73 m2. Empagliflozin should not be initiated in patients with an eGFR < 45 mL/min/1.73 m2.
The SGLT2 inhibitors are useful tools to lower blood glucose levels in people with T2DM. They can be used as monotherapy or in combination. They also cause weight reduction. Thus, their unique mechanism of action is complementary to the other oral antidiabetic medications and insulin, so a wide variety of patients can benefit from this class.
Author disclosures
Dr. Nguyen is affiliated with the Astra Zeneca Speakers Bureau and Janssen Pharmaceutical Speakers Bureau. Dr. Plodkowski is a Janssen Pharmaceutical consultant. The remaining authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.
1. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
2. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154-2161.
3. Sha S, Polidori D, Farrell, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188-197.
4. Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol. 2014;40(1):64-74.
5. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355-364.
6. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582-2592.
7. Neal B, Perkovic V, de Zeeuw D, et al; CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38(3):403-411.
8. INVOKANA [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013.
9. FARXIGA [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
10. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015-2022.
11. Rosenstock J, Hansen L, Zee P, et al. Dual Add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
12. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
13. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613-621.
14. Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2):118-123.
15. Barnett A, Mithal A, Manassie J, et al; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369-384.
16. Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedl UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215-222.
17. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators.
Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1699.
18. Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedi UC; EMPA-REG H2H-SU trial investigators. Rational, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129.
19. Kovacs CS, Seshiah V, Swallow R, et al; EMPA-REG PIO trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147-158.
20. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
21. Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33-A42.
22. Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(3):1815-1823.
23. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152-161.
24. Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331-345.
25. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90-100.
26. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213-225.
27. Macha S, Jungnik A, Hohl K, Hobson D, Salsali A, Woerle HJ. Effect of food on the pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, and assessment of dose proportionality in healthy volunteers. Int J Clinical Pharmacol Therapy. 2013;51(11):873-879.
28. Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213-219.
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721-728.
30. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPAREG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
31. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015-4021.
32. Rosenstock J, Jelaska A, Kim G, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) [Abstract 1102-P]. Diabetes. 2013;62(suppl 1):A285.
33. JARDIANCE [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals,
Inc.; 2015.
34. Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-3404.
35. FDA Drug Safety Communication: FDA Warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Updated May 19, 2015. Accessed September 23, 2015.
36. Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
37. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients
with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
38. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438-447.