User login
Study highlights need for induction strategy in elderly, frail MM patients
ATLANTA—Initial results of the phase 2 HOVON-126 trial in newly diagnosed multiple myeloma (MM) patients have highlighted the need for an induction strategy in elderly and frail patients.
The trial showed high overall response rates (ORRs) after induction with ixazomib, thalidomide, and low-dose dexamethasone.
However, 62% of patients older than 75 and 60% of frail patients discontinued therapy prior to starting maintenance.
HOVON-126 was designed to determine the ORR of induction therapy with ixazomib, thalidomide, and dexamethasone but also compare progression-free survival in patients who received ixazomib maintenance and those who received placebo.
Sonja Zweegman, MD, of VUmc in Amsterdam, The Netherlands, presented induction results from HOVON-126 at the 2017 ASH Annual Meeting (abstract 433).
The study was supported by Takeda and the Dutch Cancer Society. Dr Zweegman disclosed research funding from, and advisory board participation for, Takeda.
Study design
Investigators enrolled patients with previously untreated, symptomatic MM who were not eligible for stem cell transplant. Patients had to have measurable disease and a WHO performance status of 0 to 3 for patients younger than 75 and 0 to 2 for patients 75 or older.
Patients were not eligible if they had grade 3 neuropathy or grade 2 with pain. They were also ineligible if their creatinine clearance was less than 30 mL/minute.
All patients received ixazomib at 4 mg on days 1, 8, and 15; thalidomide at 100 mg on days 1 to 28; and dexamethasone at 40 mg on days 1, 8, 15, and 22 for nine 28-day cycles.
They could then be randomized to ixazomib maintenance (on the aforementioned schedule) or placebo for 28-day cycles until progression.
Investigators performed subgroup analyses based on cytogenetic risk and frailty.
They defined frailty according to the modified IMWG frailty index, which takes into account age, the Charlson Comorbidity Index, and the WHO performance scale as a proxy for Activities of Daily Living.
They defined high-risk cytogenetics as del17p, t(4;14), or t(14;16).
Investigators planned to enroll 142 patients and expected 94 patients to be randomized.
Patient demographics
The first 120 patients enrolled had a median age of 74 (range, 64–90). Thirty percent (n=38) were older than 75, and 8% (n=10) were older than 80.
More than two-thirds had an ISS score of I or II, and three-quarters had a WHO performance status of 0 or 1. Twenty-four percent had a performance status of 2, and 1% had a performance status of 3.
Eighty percent had lytic bone disease.
One hundred thirteen patients (94%) had FISH analysis performed. Of those, 10% had del17p, 7% had t(4;14), and 1% had t(14;16).
Eighty-one percent of patients fell into the standard-risk category and 19% into the high-risk category.
Almost half of patients (47%) were considered frail, 28% unfit, 21% fit, and 4% unknown.
Response
The ORR for induction was 81%. Ten percent of patients achieved a complete response (CR), 34% had a very good partial response (VGPR), and 37% had a partial response (PR).
The median time to response was 1.1 months, and the median time to maximum response was 4.7 months.
The response rate was independent of cytogenetic risk. Standard-risk patients achieved an ORR of 84%, a VGPR rate of 48%, and a CR rate of 10%. High-risk patients had an ORR of 79%, VGPR of 42%, and CR of 11%.
The response rate was also independent of frailty. Fit patients had an ORR of 88%, unfit patients 85%, and frail patients 75%. The VGPR rate was 36% for fit, 53% for unfit, and 43% for frail patients. The CR rate was 16% for fit, 9% for unfit, and 9% for frail patients.
Safety
“Grade 3 and 4 toxicities were found to be limited, with mainly infections, [gastrointestinal], and skin toxicity,” Dr Zweegman noted. “There was also a very low incidence of neuropathy, with only 3% grade 3 neuropathy and no grade 4 neuropathy.”
Grade 3 adverse events (AEs) occurred in 50% of patients and grade 4 in 11%.
Hematologic AEs of grade 3 and 4, respectively, included anemia (5%, 1%), thrombocytopenia (3%, 1%), and neutropenia (1%, 0).
Nonhematologic AEs of grade 3 and 4, respectively, included infections (12%, 3%), neuropathy (3%, 0), cardiac events (7%, 3%), gastrointestinal events (8%, 0), skin AEs (10%, 0), and venous thromboembolism (0, 2%).
The incidence of severe neuropathy was low. Fifty-eight percent of patients had grade 0 neuropathy, 24% grade 1, 14% grade 2, 3% grade 3, and no grade 4.
Discontinuation
Fifty-four patients (45%) discontinued therapy. The reasons for discontinuation were:
- Progressive disease, 13%
- Toxicity, 15%
- Death, 4%
- Noncompliance, 8%
- Not eligible for randomization, 0.8%
- Other, 4%.
“And when looking in detail into the toxicity, it was shown that it was mainly asthenia and neuropathy being judged by the treating physicians as caused by thalidomide,” Dr Zweegman explained.
Investigators also evaluated discontinuation according to age and found that 35% of patients 75 or younger discontinued therapy, compared with 62% of those older than 75.
However, there was no significant difference in discontinuation rate during the first 6 cycles. Seventy-seven percent of the younger patients and 69% of the older group completed 6 cycles.
Older patients who discontinued early had rates of progressive disease and toxicity comparable to the younger patients, but “there was a difference in early mortality,” Dr Zweegman added.
Nine percent of older patients discontinued before maintenance due to early mortality, compared with 1% of younger patients. And mortality in the older group was mainly due to infections and 1 cardiac arrest.
“So I think that highlights the need for antibiotic prophylaxis, which was not mandatory in this study,” Dr Zweegman said.
And finally, the investigators evaluated discontinuation according to frailty. Twenty-four percent of fit patients discontinued prior to maintenance, 32% of unfit, and 60% of frail.
Again, investigators found no significant difference in discontinuation rate during the first 6 cycles of induction. Eighty percent of fit patients completed 6 cycles, as did 79% of unfit patients and 70% of frail patients.
Despite the feasibility of the treatment and an ORR of 81%, the investigators say novel approaches are needed for frail patients and those older than 75.
“One possibility is to limit the duration of induction therapy . . . ,” Dr Zweegman said. “That would allow the start of long-term administration of maintenance treatment.”
The investigators also suggest evaluating less toxic combinations, such as ixazomib and daratumumab with lower doses of dexamethasone, the combination used in the HOVON-143 study.
Ixazomib is approved by the US Food and Drug Administration, Health Canada, and conditionally approved by the European Commission for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior therapy. ![]()
ATLANTA—Initial results of the phase 2 HOVON-126 trial in newly diagnosed multiple myeloma (MM) patients have highlighted the need for an induction strategy in elderly and frail patients.
The trial showed high overall response rates (ORRs) after induction with ixazomib, thalidomide, and low-dose dexamethasone.
However, 62% of patients older than 75 and 60% of frail patients discontinued therapy prior to starting maintenance.
HOVON-126 was designed to determine the ORR of induction therapy with ixazomib, thalidomide, and dexamethasone but also compare progression-free survival in patients who received ixazomib maintenance and those who received placebo.
Sonja Zweegman, MD, of VUmc in Amsterdam, The Netherlands, presented induction results from HOVON-126 at the 2017 ASH Annual Meeting (abstract 433).
The study was supported by Takeda and the Dutch Cancer Society. Dr Zweegman disclosed research funding from, and advisory board participation for, Takeda.
Study design
Investigators enrolled patients with previously untreated, symptomatic MM who were not eligible for stem cell transplant. Patients had to have measurable disease and a WHO performance status of 0 to 3 for patients younger than 75 and 0 to 2 for patients 75 or older.
Patients were not eligible if they had grade 3 neuropathy or grade 2 with pain. They were also ineligible if their creatinine clearance was less than 30 mL/minute.
All patients received ixazomib at 4 mg on days 1, 8, and 15; thalidomide at 100 mg on days 1 to 28; and dexamethasone at 40 mg on days 1, 8, 15, and 22 for nine 28-day cycles.
They could then be randomized to ixazomib maintenance (on the aforementioned schedule) or placebo for 28-day cycles until progression.
Investigators performed subgroup analyses based on cytogenetic risk and frailty.
They defined frailty according to the modified IMWG frailty index, which takes into account age, the Charlson Comorbidity Index, and the WHO performance scale as a proxy for Activities of Daily Living.
They defined high-risk cytogenetics as del17p, t(4;14), or t(14;16).
Investigators planned to enroll 142 patients and expected 94 patients to be randomized.
Patient demographics
The first 120 patients enrolled had a median age of 74 (range, 64–90). Thirty percent (n=38) were older than 75, and 8% (n=10) were older than 80.
More than two-thirds had an ISS score of I or II, and three-quarters had a WHO performance status of 0 or 1. Twenty-four percent had a performance status of 2, and 1% had a performance status of 3.
Eighty percent had lytic bone disease.
One hundred thirteen patients (94%) had FISH analysis performed. Of those, 10% had del17p, 7% had t(4;14), and 1% had t(14;16).
Eighty-one percent of patients fell into the standard-risk category and 19% into the high-risk category.
Almost half of patients (47%) were considered frail, 28% unfit, 21% fit, and 4% unknown.
Response
The ORR for induction was 81%. Ten percent of patients achieved a complete response (CR), 34% had a very good partial response (VGPR), and 37% had a partial response (PR).
The median time to response was 1.1 months, and the median time to maximum response was 4.7 months.
The response rate was independent of cytogenetic risk. Standard-risk patients achieved an ORR of 84%, a VGPR rate of 48%, and a CR rate of 10%. High-risk patients had an ORR of 79%, VGPR of 42%, and CR of 11%.
The response rate was also independent of frailty. Fit patients had an ORR of 88%, unfit patients 85%, and frail patients 75%. The VGPR rate was 36% for fit, 53% for unfit, and 43% for frail patients. The CR rate was 16% for fit, 9% for unfit, and 9% for frail patients.
Safety
“Grade 3 and 4 toxicities were found to be limited, with mainly infections, [gastrointestinal], and skin toxicity,” Dr Zweegman noted. “There was also a very low incidence of neuropathy, with only 3% grade 3 neuropathy and no grade 4 neuropathy.”
Grade 3 adverse events (AEs) occurred in 50% of patients and grade 4 in 11%.
Hematologic AEs of grade 3 and 4, respectively, included anemia (5%, 1%), thrombocytopenia (3%, 1%), and neutropenia (1%, 0).
Nonhematologic AEs of grade 3 and 4, respectively, included infections (12%, 3%), neuropathy (3%, 0), cardiac events (7%, 3%), gastrointestinal events (8%, 0), skin AEs (10%, 0), and venous thromboembolism (0, 2%).
The incidence of severe neuropathy was low. Fifty-eight percent of patients had grade 0 neuropathy, 24% grade 1, 14% grade 2, 3% grade 3, and no grade 4.
Discontinuation
Fifty-four patients (45%) discontinued therapy. The reasons for discontinuation were:
- Progressive disease, 13%
- Toxicity, 15%
- Death, 4%
- Noncompliance, 8%
- Not eligible for randomization, 0.8%
- Other, 4%.
“And when looking in detail into the toxicity, it was shown that it was mainly asthenia and neuropathy being judged by the treating physicians as caused by thalidomide,” Dr Zweegman explained.
Investigators also evaluated discontinuation according to age and found that 35% of patients 75 or younger discontinued therapy, compared with 62% of those older than 75.
However, there was no significant difference in discontinuation rate during the first 6 cycles. Seventy-seven percent of the younger patients and 69% of the older group completed 6 cycles.
Older patients who discontinued early had rates of progressive disease and toxicity comparable to the younger patients, but “there was a difference in early mortality,” Dr Zweegman added.
Nine percent of older patients discontinued before maintenance due to early mortality, compared with 1% of younger patients. And mortality in the older group was mainly due to infections and 1 cardiac arrest.
“So I think that highlights the need for antibiotic prophylaxis, which was not mandatory in this study,” Dr Zweegman said.
And finally, the investigators evaluated discontinuation according to frailty. Twenty-four percent of fit patients discontinued prior to maintenance, 32% of unfit, and 60% of frail.
Again, investigators found no significant difference in discontinuation rate during the first 6 cycles of induction. Eighty percent of fit patients completed 6 cycles, as did 79% of unfit patients and 70% of frail patients.
Despite the feasibility of the treatment and an ORR of 81%, the investigators say novel approaches are needed for frail patients and those older than 75.
“One possibility is to limit the duration of induction therapy . . . ,” Dr Zweegman said. “That would allow the start of long-term administration of maintenance treatment.”
The investigators also suggest evaluating less toxic combinations, such as ixazomib and daratumumab with lower doses of dexamethasone, the combination used in the HOVON-143 study.
Ixazomib is approved by the US Food and Drug Administration, Health Canada, and conditionally approved by the European Commission for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior therapy. ![]()
ATLANTA—Initial results of the phase 2 HOVON-126 trial in newly diagnosed multiple myeloma (MM) patients have highlighted the need for an induction strategy in elderly and frail patients.
The trial showed high overall response rates (ORRs) after induction with ixazomib, thalidomide, and low-dose dexamethasone.
However, 62% of patients older than 75 and 60% of frail patients discontinued therapy prior to starting maintenance.
HOVON-126 was designed to determine the ORR of induction therapy with ixazomib, thalidomide, and dexamethasone but also compare progression-free survival in patients who received ixazomib maintenance and those who received placebo.
Sonja Zweegman, MD, of VUmc in Amsterdam, The Netherlands, presented induction results from HOVON-126 at the 2017 ASH Annual Meeting (abstract 433).
The study was supported by Takeda and the Dutch Cancer Society. Dr Zweegman disclosed research funding from, and advisory board participation for, Takeda.
Study design
Investigators enrolled patients with previously untreated, symptomatic MM who were not eligible for stem cell transplant. Patients had to have measurable disease and a WHO performance status of 0 to 3 for patients younger than 75 and 0 to 2 for patients 75 or older.
Patients were not eligible if they had grade 3 neuropathy or grade 2 with pain. They were also ineligible if their creatinine clearance was less than 30 mL/minute.
All patients received ixazomib at 4 mg on days 1, 8, and 15; thalidomide at 100 mg on days 1 to 28; and dexamethasone at 40 mg on days 1, 8, 15, and 22 for nine 28-day cycles.
They could then be randomized to ixazomib maintenance (on the aforementioned schedule) or placebo for 28-day cycles until progression.
Investigators performed subgroup analyses based on cytogenetic risk and frailty.
They defined frailty according to the modified IMWG frailty index, which takes into account age, the Charlson Comorbidity Index, and the WHO performance scale as a proxy for Activities of Daily Living.
They defined high-risk cytogenetics as del17p, t(4;14), or t(14;16).
Investigators planned to enroll 142 patients and expected 94 patients to be randomized.
Patient demographics
The first 120 patients enrolled had a median age of 74 (range, 64–90). Thirty percent (n=38) were older than 75, and 8% (n=10) were older than 80.
More than two-thirds had an ISS score of I or II, and three-quarters had a WHO performance status of 0 or 1. Twenty-four percent had a performance status of 2, and 1% had a performance status of 3.
Eighty percent had lytic bone disease.
One hundred thirteen patients (94%) had FISH analysis performed. Of those, 10% had del17p, 7% had t(4;14), and 1% had t(14;16).
Eighty-one percent of patients fell into the standard-risk category and 19% into the high-risk category.
Almost half of patients (47%) were considered frail, 28% unfit, 21% fit, and 4% unknown.
Response
The ORR for induction was 81%. Ten percent of patients achieved a complete response (CR), 34% had a very good partial response (VGPR), and 37% had a partial response (PR).
The median time to response was 1.1 months, and the median time to maximum response was 4.7 months.
The response rate was independent of cytogenetic risk. Standard-risk patients achieved an ORR of 84%, a VGPR rate of 48%, and a CR rate of 10%. High-risk patients had an ORR of 79%, VGPR of 42%, and CR of 11%.
The response rate was also independent of frailty. Fit patients had an ORR of 88%, unfit patients 85%, and frail patients 75%. The VGPR rate was 36% for fit, 53% for unfit, and 43% for frail patients. The CR rate was 16% for fit, 9% for unfit, and 9% for frail patients.
Safety
“Grade 3 and 4 toxicities were found to be limited, with mainly infections, [gastrointestinal], and skin toxicity,” Dr Zweegman noted. “There was also a very low incidence of neuropathy, with only 3% grade 3 neuropathy and no grade 4 neuropathy.”
Grade 3 adverse events (AEs) occurred in 50% of patients and grade 4 in 11%.
Hematologic AEs of grade 3 and 4, respectively, included anemia (5%, 1%), thrombocytopenia (3%, 1%), and neutropenia (1%, 0).
Nonhematologic AEs of grade 3 and 4, respectively, included infections (12%, 3%), neuropathy (3%, 0), cardiac events (7%, 3%), gastrointestinal events (8%, 0), skin AEs (10%, 0), and venous thromboembolism (0, 2%).
The incidence of severe neuropathy was low. Fifty-eight percent of patients had grade 0 neuropathy, 24% grade 1, 14% grade 2, 3% grade 3, and no grade 4.
Discontinuation
Fifty-four patients (45%) discontinued therapy. The reasons for discontinuation were:
- Progressive disease, 13%
- Toxicity, 15%
- Death, 4%
- Noncompliance, 8%
- Not eligible for randomization, 0.8%
- Other, 4%.
“And when looking in detail into the toxicity, it was shown that it was mainly asthenia and neuropathy being judged by the treating physicians as caused by thalidomide,” Dr Zweegman explained.
Investigators also evaluated discontinuation according to age and found that 35% of patients 75 or younger discontinued therapy, compared with 62% of those older than 75.
However, there was no significant difference in discontinuation rate during the first 6 cycles. Seventy-seven percent of the younger patients and 69% of the older group completed 6 cycles.
Older patients who discontinued early had rates of progressive disease and toxicity comparable to the younger patients, but “there was a difference in early mortality,” Dr Zweegman added.
Nine percent of older patients discontinued before maintenance due to early mortality, compared with 1% of younger patients. And mortality in the older group was mainly due to infections and 1 cardiac arrest.
“So I think that highlights the need for antibiotic prophylaxis, which was not mandatory in this study,” Dr Zweegman said.
And finally, the investigators evaluated discontinuation according to frailty. Twenty-four percent of fit patients discontinued prior to maintenance, 32% of unfit, and 60% of frail.
Again, investigators found no significant difference in discontinuation rate during the first 6 cycles of induction. Eighty percent of fit patients completed 6 cycles, as did 79% of unfit patients and 70% of frail patients.
Despite the feasibility of the treatment and an ORR of 81%, the investigators say novel approaches are needed for frail patients and those older than 75.
“One possibility is to limit the duration of induction therapy . . . ,” Dr Zweegman said. “That would allow the start of long-term administration of maintenance treatment.”
The investigators also suggest evaluating less toxic combinations, such as ixazomib and daratumumab with lower doses of dexamethasone, the combination used in the HOVON-143 study.
Ixazomib is approved by the US Food and Drug Administration, Health Canada, and conditionally approved by the European Commission for use in combination with lenalidomide and dexamethasone to treat MM patients who have received at least 1 prior therapy. ![]()
Survival improvements lag for young Hispanic patients with myeloma
ATLANTA –
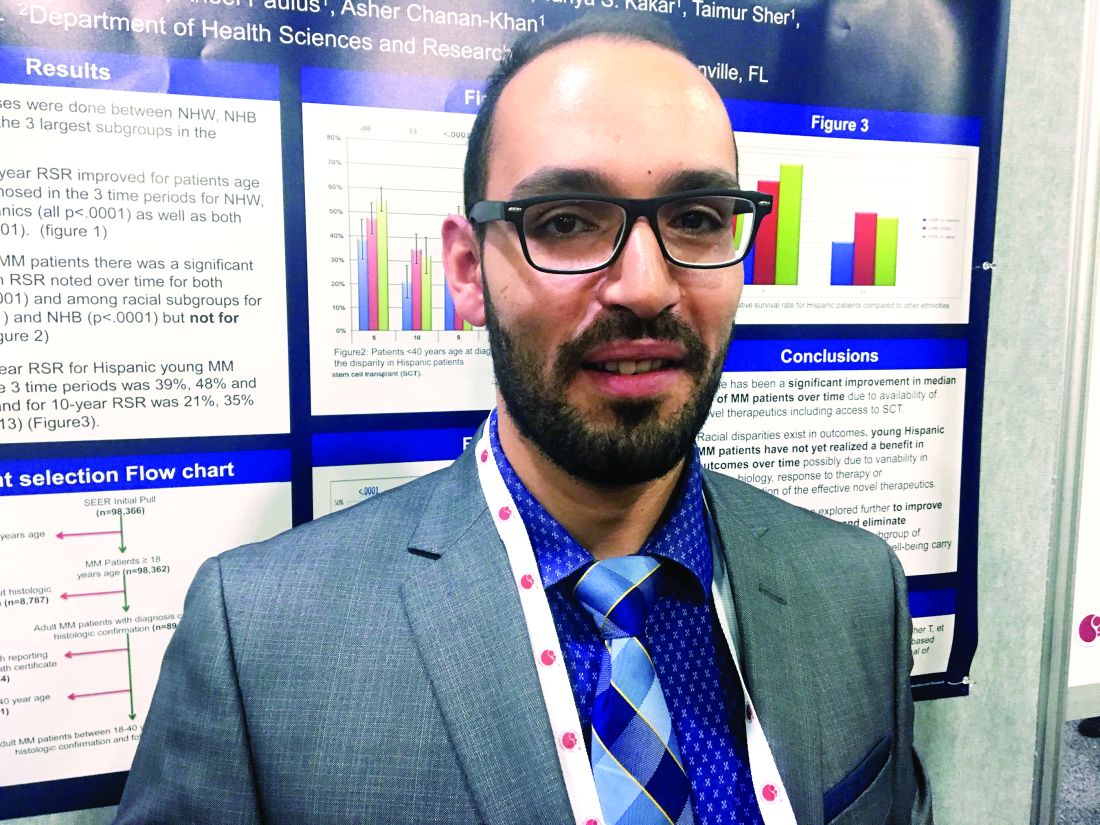
Among U.S. adults diagnosed with multiple myeloma by age 40 years, 5-year and 10-year survival improved significantly (P less than .0001) for non-Hispanic blacks and whites, but not for Hispanics (5-year survival, P = .08; 10-year survival, P = .13), Abdel-Ghani Azzouqa, MD, and colleagues reported in a poster at the annual meeting of the American Society of Hematology.
Other population-based studies have uncovered racial and ethnic disparities in myeloma outcomes but had not honed in on the experience of young adult patients, who make up a growing proportion of diagnosed patients, said Dr. Azzouqa.
He and his associates analyzed Surveillance Epidemiology and End Results (SEER) data on patients diagnosed between ages 18 and 40 years with histologically confirmed multiple myeloma. The dataset spanned 1973-2014 and included 1,460 patients, of whom about 60% were male. Median age at diagnosis was 37 years; 47% of patients were non-Hispanic white, 28% were non-Hispanic black, 18% were Hispanic, 5.5% were Asian, and about 1% were of other ethnicities.
For young Hispanic patients with myeloma, 5-year survival improved from 39% before 1996, when stem cell transplants and novel therapies became available, to 56% from 2002 onward. This change was not statistically significant (P = .08), and 10-year survival rates also did not change significantly (from 21% to 33%; P = .13).
Five-year and 10-year survival did improve significantly for both genders (P = .0001) and among non-Hispanic blacks (P = .0001) and non-Hispanic whites (P = .0001).
Racial/ethnic subgroups did not differ significantly by median age at diagnosis, gender distribution, or listed cause of death, Dr. Azzouqa noted. Thus, reasons for the difference in survival for Hispanic patients remain unclear. Perhaps they reflect differences in disease biology, treatment response, or access or use of effective novel therapies, he said.
The researchers had no external funding sources. Dr. Azzouqa had no conflicts of interest. Lead author Dr. Sikander Ailawadhi disclosed ties to funding Pharmacyclics, Amgen, Novartis, and Takeda.
SOURCE: Ailawadhi S et al. ASH Abstract 2149
ATLANTA –
Among U.S. adults diagnosed with multiple myeloma by age 40 years, 5-year and 10-year survival improved significantly (P less than .0001) for non-Hispanic blacks and whites, but not for Hispanics (5-year survival, P = .08; 10-year survival, P = .13), Abdel-Ghani Azzouqa, MD, and colleagues reported in a poster at the annual meeting of the American Society of Hematology.
Other population-based studies have uncovered racial and ethnic disparities in myeloma outcomes but had not honed in on the experience of young adult patients, who make up a growing proportion of diagnosed patients, said Dr. Azzouqa.
He and his associates analyzed Surveillance Epidemiology and End Results (SEER) data on patients diagnosed between ages 18 and 40 years with histologically confirmed multiple myeloma. The dataset spanned 1973-2014 and included 1,460 patients, of whom about 60% were male. Median age at diagnosis was 37 years; 47% of patients were non-Hispanic white, 28% were non-Hispanic black, 18% were Hispanic, 5.5% were Asian, and about 1% were of other ethnicities.
For young Hispanic patients with myeloma, 5-year survival improved from 39% before 1996, when stem cell transplants and novel therapies became available, to 56% from 2002 onward. This change was not statistically significant (P = .08), and 10-year survival rates also did not change significantly (from 21% to 33%; P = .13).
Five-year and 10-year survival did improve significantly for both genders (P = .0001) and among non-Hispanic blacks (P = .0001) and non-Hispanic whites (P = .0001).
Racial/ethnic subgroups did not differ significantly by median age at diagnosis, gender distribution, or listed cause of death, Dr. Azzouqa noted. Thus, reasons for the difference in survival for Hispanic patients remain unclear. Perhaps they reflect differences in disease biology, treatment response, or access or use of effective novel therapies, he said.
The researchers had no external funding sources. Dr. Azzouqa had no conflicts of interest. Lead author Dr. Sikander Ailawadhi disclosed ties to funding Pharmacyclics, Amgen, Novartis, and Takeda.
SOURCE: Ailawadhi S et al. ASH Abstract 2149
ATLANTA –
Among U.S. adults diagnosed with multiple myeloma by age 40 years, 5-year and 10-year survival improved significantly (P less than .0001) for non-Hispanic blacks and whites, but not for Hispanics (5-year survival, P = .08; 10-year survival, P = .13), Abdel-Ghani Azzouqa, MD, and colleagues reported in a poster at the annual meeting of the American Society of Hematology.
Other population-based studies have uncovered racial and ethnic disparities in myeloma outcomes but had not honed in on the experience of young adult patients, who make up a growing proportion of diagnosed patients, said Dr. Azzouqa.
He and his associates analyzed Surveillance Epidemiology and End Results (SEER) data on patients diagnosed between ages 18 and 40 years with histologically confirmed multiple myeloma. The dataset spanned 1973-2014 and included 1,460 patients, of whom about 60% were male. Median age at diagnosis was 37 years; 47% of patients were non-Hispanic white, 28% were non-Hispanic black, 18% were Hispanic, 5.5% were Asian, and about 1% were of other ethnicities.
For young Hispanic patients with myeloma, 5-year survival improved from 39% before 1996, when stem cell transplants and novel therapies became available, to 56% from 2002 onward. This change was not statistically significant (P = .08), and 10-year survival rates also did not change significantly (from 21% to 33%; P = .13).
Five-year and 10-year survival did improve significantly for both genders (P = .0001) and among non-Hispanic blacks (P = .0001) and non-Hispanic whites (P = .0001).
Racial/ethnic subgroups did not differ significantly by median age at diagnosis, gender distribution, or listed cause of death, Dr. Azzouqa noted. Thus, reasons for the difference in survival for Hispanic patients remain unclear. Perhaps they reflect differences in disease biology, treatment response, or access or use of effective novel therapies, he said.
The researchers had no external funding sources. Dr. Azzouqa had no conflicts of interest. Lead author Dr. Sikander Ailawadhi disclosed ties to funding Pharmacyclics, Amgen, Novartis, and Takeda.
SOURCE: Ailawadhi S et al. ASH Abstract 2149
REPORTING FROM ASH 2017
Key clinical point: Recent improvements in multiple myeloma survival have left young Hispanics behind.
Major finding: Five-year and 10-year survival have improved significantly among young blacks and non-Hispanic whites with multiple myeloma (P less than .0001 for all comparisons) but not Hispanics (5-year survival P = .08; 10-year survival P = .13).
Data source: Surveillance Epidemiology and End Results (SEER) data for 1,460 adults up to 40 years old when diagnosed with multiple myeloma.
Disclosures: The researchers had no external funding sources. Dr. Azzouqa had no conflicts of interest. Lead author Dr. Sikander Ailawadhi disclosed funding from Pharmacyclics, Amgen, Novartis, and Takeda.
Source: Ailawadhi S et al. ASH Abstract 2149.
Venetoclax/rituximab boosts PFS in relapsed/refractory CLL
ATLANTA – In patients with relapsed/refractory chronic lymphocytic leukemia (CLL), a combination of venetoclax (Venclexta) and rituximab was superior to bendamustine (Treanda) and rituximab for prolonging progression-free survival (PFS), with effects consistent across subgroups, regardless of mutational status, and a clinically meaningful improvement in overall survival.
An interim analysis from the phase 3 MURANO trial showed that after a median follow-up of 23.8 months, the median PFS for patients randomized to venetoclax/rituximab had not been reached, compared with 17 months for patients assigned to bendamustine/rituximab, reported John F. Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre at the University of Melbourne.
Relapsed/refractory CLL often has a suboptimal response to conventional chemotherapy because of adverse biological features that can accumulate in cells, he said.
The combination of bendamustine and rituximab has been associated with about 60% overall responses rates, a median PFS of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, he noted.
The rationale for pairing venetoclax with rituximab in this population comes from evidence showing efficacy of the monoclonal antibody, an oral B-cell lymphoma–2 (BCL-2) inhibitor, as monotherapy in patients with relapsed/refractory CLL, including those with poor prognostic features such as the 17p deletion (del17p).
Dr. Seymour and his colleagues recently published results from a phase 1b trial of venetoclax/rituximab in patients with relapsed/refractory CLL. The combination was associated with a 51% complete response rate, and a 28% rate of negative marrow minimal residual disease (MRD) (Lancet Oncol. 2017 Feb;18[2]:230-40)
In the MURANO study (NCT02005471), the investigators evaluated whether time-limited therapy with venetoclax/rituximab could improve PFS over bendamustine/rituximab.
Patients 18 and older with CLL who had been treated with one to three prior lines of therapy, including at least one chemotherapy-containing regimen, were enrolled. Prior treatment with bendamustine was allowed only if patients had had a duration of response of at least 24 months.
After stratification by del17p status, responsiveness to prior therapy, and geographic region, 389 patients were randomly assigned to receive rituximab 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of cycles 2 through 6, plus either bendamustine 70 mg/m2 on days 1 and 2 of each of six cycles, or venetoclax 400 mg orally once daily until disease progression, cessation for toxicity, or up to a maximum of 2 years starting from day 1 of cycle 1.
As noted,
The respective 1- and 2-year PFS rates with venetoclax were 91.2% and 82.8%, compared with 74.1% and 37.4% with bendamustine.
The venetoclax/rituximab combination was also significantly superior across all subgroups, regardless of the number of prior therapies, refractory vs. relapsed after most recent prior therapy, del17p status, TP53 mutational status, or baseline immunoglobulin heavy chain variable (IGHV) mutated or unmutated status
Response rates assessed by both investigators and independent reviewers were also better with venetoclax. The investigator-assessed overall response rate (ORR) was 93.3%, compared with 67.7% for bendamustine/rituximab, including 26.8% complete responses (CR), compared with 8.2%. Independent reviewers decreed an ORR of 92.3% for venentoclax, vs. 72.3% for bendamustine, including respective CR rates of 8.2% and 3.6%.
The investigators also found that the percentage of MRD negativity was higher with venetoclax/rituximab, with 62% of patients in this group being MRD negative at 9 months. This rate remained fairly constant at 12-, 15- and 18-month follow-ups (60%, 57%, and 60%, respectively).
In contrast, 13% of patients treated with bendamustine were MRD negative at 9 months, and the rates gradually declined over time to 10%, 9%, and 5%.
Investigators also saw a clinically meaningful improvement in overall survival with the venetoclax/rituximab duo, although survival data are still not mature in this ongoing trial. The median OS had not been reached in either group at the time of data cutoff.
Respective 1- and 2-year OS rates with venetoclax were 95.9% and 91.9%, and with bendamustine were 91.1% and 86.6%.
At the time of this interim analysis, the hazard ratio favoring venetoclax/rituximab was 0.48 (P = .0186).
Drug discontinuation was more frequent with venetoclax/rituximab (25% vs, 17%), with disease progression and adverse events without progression being the most frequent reasons for stopping in each arm.
Serious adverse events occurred in 46% of patients on venetoclax/rituximab and 43% on bendamustine/rituximab. A higher percentage of patients on venetoclax/rituximab had grade 3 or 4 adverse events (82% vs, 70%). Ten patients (5%) in the venetoclax/rituximab arm died, and 11 patients (6%) on bendamustine/rituximab died.
Events with a greater than 2% difference included more frequent neutropenia, tumor lysis syndrome, hyperglycemia and hypogammaglobulinema with venetoclax/rituximab, and more frequent anemia, thrombocytopenia, febrile neutropenia, pneumonia, infusion-related reactions, and hypotension with bendamustine/rituximab.
In the question-and-response portion following Dr. Seymour’s presentation, an audience member commented that the continuation of venetoclax/rituximab beyond the initial treatment cycles amounted to a maintenance strategy, and that patients in the experimental arm were in treatment longer, which likely influenced the results.
“You’re absolutely correct that the treatment duration differed, although, of course, the capacity to deliver more than six cycles of bendamustine/rituximab would have been problematic,” Dr. Seymour replied.
“There are some data that antibody treatment may prolong progression-free survival. However, when this study was designed in 2013 that data was certainly not available, and I believe currently even maintenance antibodies are not an accepted standard of treatment,” he added.
The MURANO trial was funded by AbbVie and Genentech. Dr. Seymour disclosed honoraria, speakers bureau, research funding, and advisory activities with AbbVie and other companies.
SOURCE: Seymour J et al. ASH 2017 LBA-2.
ATLANTA – In patients with relapsed/refractory chronic lymphocytic leukemia (CLL), a combination of venetoclax (Venclexta) and rituximab was superior to bendamustine (Treanda) and rituximab for prolonging progression-free survival (PFS), with effects consistent across subgroups, regardless of mutational status, and a clinically meaningful improvement in overall survival.
An interim analysis from the phase 3 MURANO trial showed that after a median follow-up of 23.8 months, the median PFS for patients randomized to venetoclax/rituximab had not been reached, compared with 17 months for patients assigned to bendamustine/rituximab, reported John F. Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre at the University of Melbourne.
Relapsed/refractory CLL often has a suboptimal response to conventional chemotherapy because of adverse biological features that can accumulate in cells, he said.
The combination of bendamustine and rituximab has been associated with about 60% overall responses rates, a median PFS of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, he noted.
The rationale for pairing venetoclax with rituximab in this population comes from evidence showing efficacy of the monoclonal antibody, an oral B-cell lymphoma–2 (BCL-2) inhibitor, as monotherapy in patients with relapsed/refractory CLL, including those with poor prognostic features such as the 17p deletion (del17p).
Dr. Seymour and his colleagues recently published results from a phase 1b trial of venetoclax/rituximab in patients with relapsed/refractory CLL. The combination was associated with a 51% complete response rate, and a 28% rate of negative marrow minimal residual disease (MRD) (Lancet Oncol. 2017 Feb;18[2]:230-40)
In the MURANO study (NCT02005471), the investigators evaluated whether time-limited therapy with venetoclax/rituximab could improve PFS over bendamustine/rituximab.
Patients 18 and older with CLL who had been treated with one to three prior lines of therapy, including at least one chemotherapy-containing regimen, were enrolled. Prior treatment with bendamustine was allowed only if patients had had a duration of response of at least 24 months.
After stratification by del17p status, responsiveness to prior therapy, and geographic region, 389 patients were randomly assigned to receive rituximab 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of cycles 2 through 6, plus either bendamustine 70 mg/m2 on days 1 and 2 of each of six cycles, or venetoclax 400 mg orally once daily until disease progression, cessation for toxicity, or up to a maximum of 2 years starting from day 1 of cycle 1.
As noted,
The respective 1- and 2-year PFS rates with venetoclax were 91.2% and 82.8%, compared with 74.1% and 37.4% with bendamustine.
The venetoclax/rituximab combination was also significantly superior across all subgroups, regardless of the number of prior therapies, refractory vs. relapsed after most recent prior therapy, del17p status, TP53 mutational status, or baseline immunoglobulin heavy chain variable (IGHV) mutated or unmutated status
Response rates assessed by both investigators and independent reviewers were also better with venetoclax. The investigator-assessed overall response rate (ORR) was 93.3%, compared with 67.7% for bendamustine/rituximab, including 26.8% complete responses (CR), compared with 8.2%. Independent reviewers decreed an ORR of 92.3% for venentoclax, vs. 72.3% for bendamustine, including respective CR rates of 8.2% and 3.6%.
The investigators also found that the percentage of MRD negativity was higher with venetoclax/rituximab, with 62% of patients in this group being MRD negative at 9 months. This rate remained fairly constant at 12-, 15- and 18-month follow-ups (60%, 57%, and 60%, respectively).
In contrast, 13% of patients treated with bendamustine were MRD negative at 9 months, and the rates gradually declined over time to 10%, 9%, and 5%.
Investigators also saw a clinically meaningful improvement in overall survival with the venetoclax/rituximab duo, although survival data are still not mature in this ongoing trial. The median OS had not been reached in either group at the time of data cutoff.
Respective 1- and 2-year OS rates with venetoclax were 95.9% and 91.9%, and with bendamustine were 91.1% and 86.6%.
At the time of this interim analysis, the hazard ratio favoring venetoclax/rituximab was 0.48 (P = .0186).
Drug discontinuation was more frequent with venetoclax/rituximab (25% vs, 17%), with disease progression and adverse events without progression being the most frequent reasons for stopping in each arm.
Serious adverse events occurred in 46% of patients on venetoclax/rituximab and 43% on bendamustine/rituximab. A higher percentage of patients on venetoclax/rituximab had grade 3 or 4 adverse events (82% vs, 70%). Ten patients (5%) in the venetoclax/rituximab arm died, and 11 patients (6%) on bendamustine/rituximab died.
Events with a greater than 2% difference included more frequent neutropenia, tumor lysis syndrome, hyperglycemia and hypogammaglobulinema with venetoclax/rituximab, and more frequent anemia, thrombocytopenia, febrile neutropenia, pneumonia, infusion-related reactions, and hypotension with bendamustine/rituximab.
In the question-and-response portion following Dr. Seymour’s presentation, an audience member commented that the continuation of venetoclax/rituximab beyond the initial treatment cycles amounted to a maintenance strategy, and that patients in the experimental arm were in treatment longer, which likely influenced the results.
“You’re absolutely correct that the treatment duration differed, although, of course, the capacity to deliver more than six cycles of bendamustine/rituximab would have been problematic,” Dr. Seymour replied.
“There are some data that antibody treatment may prolong progression-free survival. However, when this study was designed in 2013 that data was certainly not available, and I believe currently even maintenance antibodies are not an accepted standard of treatment,” he added.
The MURANO trial was funded by AbbVie and Genentech. Dr. Seymour disclosed honoraria, speakers bureau, research funding, and advisory activities with AbbVie and other companies.
SOURCE: Seymour J et al. ASH 2017 LBA-2.
ATLANTA – In patients with relapsed/refractory chronic lymphocytic leukemia (CLL), a combination of venetoclax (Venclexta) and rituximab was superior to bendamustine (Treanda) and rituximab for prolonging progression-free survival (PFS), with effects consistent across subgroups, regardless of mutational status, and a clinically meaningful improvement in overall survival.
An interim analysis from the phase 3 MURANO trial showed that after a median follow-up of 23.8 months, the median PFS for patients randomized to venetoclax/rituximab had not been reached, compared with 17 months for patients assigned to bendamustine/rituximab, reported John F. Seymour, MBBS, PhD, of the Peter MacCallum Cancer Centre at the University of Melbourne.
Relapsed/refractory CLL often has a suboptimal response to conventional chemotherapy because of adverse biological features that can accumulate in cells, he said.
The combination of bendamustine and rituximab has been associated with about 60% overall responses rates, a median PFS of approximately 15 months, and overall survival of nearly 3 years in patients with CLL, he noted.
The rationale for pairing venetoclax with rituximab in this population comes from evidence showing efficacy of the monoclonal antibody, an oral B-cell lymphoma–2 (BCL-2) inhibitor, as monotherapy in patients with relapsed/refractory CLL, including those with poor prognostic features such as the 17p deletion (del17p).
Dr. Seymour and his colleagues recently published results from a phase 1b trial of venetoclax/rituximab in patients with relapsed/refractory CLL. The combination was associated with a 51% complete response rate, and a 28% rate of negative marrow minimal residual disease (MRD) (Lancet Oncol. 2017 Feb;18[2]:230-40)
In the MURANO study (NCT02005471), the investigators evaluated whether time-limited therapy with venetoclax/rituximab could improve PFS over bendamustine/rituximab.
Patients 18 and older with CLL who had been treated with one to three prior lines of therapy, including at least one chemotherapy-containing regimen, were enrolled. Prior treatment with bendamustine was allowed only if patients had had a duration of response of at least 24 months.
After stratification by del17p status, responsiveness to prior therapy, and geographic region, 389 patients were randomly assigned to receive rituximab 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of cycles 2 through 6, plus either bendamustine 70 mg/m2 on days 1 and 2 of each of six cycles, or venetoclax 400 mg orally once daily until disease progression, cessation for toxicity, or up to a maximum of 2 years starting from day 1 of cycle 1.
As noted,
The respective 1- and 2-year PFS rates with venetoclax were 91.2% and 82.8%, compared with 74.1% and 37.4% with bendamustine.
The venetoclax/rituximab combination was also significantly superior across all subgroups, regardless of the number of prior therapies, refractory vs. relapsed after most recent prior therapy, del17p status, TP53 mutational status, or baseline immunoglobulin heavy chain variable (IGHV) mutated or unmutated status
Response rates assessed by both investigators and independent reviewers were also better with venetoclax. The investigator-assessed overall response rate (ORR) was 93.3%, compared with 67.7% for bendamustine/rituximab, including 26.8% complete responses (CR), compared with 8.2%. Independent reviewers decreed an ORR of 92.3% for venentoclax, vs. 72.3% for bendamustine, including respective CR rates of 8.2% and 3.6%.
The investigators also found that the percentage of MRD negativity was higher with venetoclax/rituximab, with 62% of patients in this group being MRD negative at 9 months. This rate remained fairly constant at 12-, 15- and 18-month follow-ups (60%, 57%, and 60%, respectively).
In contrast, 13% of patients treated with bendamustine were MRD negative at 9 months, and the rates gradually declined over time to 10%, 9%, and 5%.
Investigators also saw a clinically meaningful improvement in overall survival with the venetoclax/rituximab duo, although survival data are still not mature in this ongoing trial. The median OS had not been reached in either group at the time of data cutoff.
Respective 1- and 2-year OS rates with venetoclax were 95.9% and 91.9%, and with bendamustine were 91.1% and 86.6%.
At the time of this interim analysis, the hazard ratio favoring venetoclax/rituximab was 0.48 (P = .0186).
Drug discontinuation was more frequent with venetoclax/rituximab (25% vs, 17%), with disease progression and adverse events without progression being the most frequent reasons for stopping in each arm.
Serious adverse events occurred in 46% of patients on venetoclax/rituximab and 43% on bendamustine/rituximab. A higher percentage of patients on venetoclax/rituximab had grade 3 or 4 adverse events (82% vs, 70%). Ten patients (5%) in the venetoclax/rituximab arm died, and 11 patients (6%) on bendamustine/rituximab died.
Events with a greater than 2% difference included more frequent neutropenia, tumor lysis syndrome, hyperglycemia and hypogammaglobulinema with venetoclax/rituximab, and more frequent anemia, thrombocytopenia, febrile neutropenia, pneumonia, infusion-related reactions, and hypotension with bendamustine/rituximab.
In the question-and-response portion following Dr. Seymour’s presentation, an audience member commented that the continuation of venetoclax/rituximab beyond the initial treatment cycles amounted to a maintenance strategy, and that patients in the experimental arm were in treatment longer, which likely influenced the results.
“You’re absolutely correct that the treatment duration differed, although, of course, the capacity to deliver more than six cycles of bendamustine/rituximab would have been problematic,” Dr. Seymour replied.
“There are some data that antibody treatment may prolong progression-free survival. However, when this study was designed in 2013 that data was certainly not available, and I believe currently even maintenance antibodies are not an accepted standard of treatment,” he added.
The MURANO trial was funded by AbbVie and Genentech. Dr. Seymour disclosed honoraria, speakers bureau, research funding, and advisory activities with AbbVie and other companies.
SOURCE: Seymour J et al. ASH 2017 LBA-2.
REPORTING FROM ASH 2017
Key clinical point: Compared with bendamustine/rituximab, venetoclax/rituximab was associated with significantly superior progression-free survival of relapsed/refractory chronic lymphocytic leukemia.
Major finding: The hazard ratio for PFS with venetoclax/rituximab was 0.17 (P less than .001).
Data source: A randomized phase 3, open-label trial in 389 patients with relapsed/refractory CLL.
Disclosures: The MURANO trial was funded by AbbVie and Genetech. Dr. Seymour disclosed honoraria, speakers bureau, research funding, and advisory activities with AbbVie and other companies.
Source: Seymour J et al. ASH 2017 LBA-2.
Team develops new scoring systems for PMF
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
Single-agent daratumumab active in smoldering multiple myeloma
ATLANTA – Daratumumab monotherapy led to durable partial responses among intermediate to high-risk patients with smoldering multiple myeloma, according to results from the phase II CENTAURUS trial.
Although less than 5% of patients had complete responses, 27% had at least a very good partial response to long-term therapy (up to 20 treatment cycles lasting 8 weeks each), Craig C. Hofmeister, MD, of the Ohio State University Comprehensive Cancer Center, Columbus, said at the annual meeting of the American Society of Hematology. The coprimary endpoint, median progression-free survival, exceeded 24 months in all dose cohorts, and was the longest when patients were treated longest.
Current guidelines recommend monitoring smoldering multiple myeloma every 3-6 months and treating only after patients progress. However, some experts pursue earlier treatment in the premalignant setting.
In CENTAURUS, 123 adults with smoldering multiple myeloma were randomly assigned to receive daratumumab (16 mg/kg IV) in 8-week cycles according to a long, intermediate, or short/intense schedule. The long schedule consisted of treatment weekly for cycle 1, every other week for cycles 2-3, monthly for cycles 4-7, and once every 8 weeks for up to 13 more cycles. The intermediate schedule consisted of treatment weekly in cycle 1 and every 8 weeks for up to 20 cycles. The short, intense schedule consisted of weekly treatment for 8 weeks (one cycle). Patients were followed for up to 4 years or until they progressed to multiple myeloma based on International Myeloma Working Group guidelines.
Over a median follow-up period of 15.8 months (range, 0 to 24 months), rates of complete response were 2% in the long treatment arm, 5% in the intermediate treatment arm, and 0% in the short treatment arm. Rates of at least very good partial response were 29%, 24%, and 15%, respectively. Overall response rates were 56%, 54%, and 38%, respectively. Median PFS was not reached in any arm, exceeding 24 months.
Treatment was generally well tolerated, said Dr. Hofmeister. The most common treatment-related adverse effects were fatigue, cough, upper respiratory tract infection, headache, and insomnia. Hypertension and hyperglycemia were the most common grade 3-4 treatment-emergent adverse events, affecting up to 5% of patients per arm. Fewer than 10% of patients in any arm developed treatment-emergent hematologic adverse events, and fewer than 5% developed grade 3-4 pneumonia or sepsis. There were three cases of a second primary malignancy, including one case of breast cancer and two cases of melanoma.
Rates of infusion-related reactions did not correlate with treatment duration. Grade 3-4 infusion-related reactions affected 0% to 3% of patients per arm. The sole death in this trial resulted from disease progression in a patient from the short treatment arm. “Taken together, efficacy and safety data support long dosing compared to intermediate and short dosing,” Dr. Hofmeister said.
The three arms were demographically similar. Patients tended to be white, in their late 50s to 60s, and to have ECOG scores of 0 with at least two risk factors for progression. About 70% had IgG disease and nearly half had less than 20% plasma cells in bone marrow.
Janssen, the maker of daratumumab, sponsored the trial. Dr. Hofmeister disclosed research funding from Janssen and research support, honoraria, and advisory relationships with Adaptive Biotechnologies, Thrasos, Celgene, Karyopharm, Takeda, and other pharmaceutical companies.
SOURCE: Hofmeister C et al, ASH 2017, Abstract 510.
ATLANTA – Daratumumab monotherapy led to durable partial responses among intermediate to high-risk patients with smoldering multiple myeloma, according to results from the phase II CENTAURUS trial.
Although less than 5% of patients had complete responses, 27% had at least a very good partial response to long-term therapy (up to 20 treatment cycles lasting 8 weeks each), Craig C. Hofmeister, MD, of the Ohio State University Comprehensive Cancer Center, Columbus, said at the annual meeting of the American Society of Hematology. The coprimary endpoint, median progression-free survival, exceeded 24 months in all dose cohorts, and was the longest when patients were treated longest.
Current guidelines recommend monitoring smoldering multiple myeloma every 3-6 months and treating only after patients progress. However, some experts pursue earlier treatment in the premalignant setting.
In CENTAURUS, 123 adults with smoldering multiple myeloma were randomly assigned to receive daratumumab (16 mg/kg IV) in 8-week cycles according to a long, intermediate, or short/intense schedule. The long schedule consisted of treatment weekly for cycle 1, every other week for cycles 2-3, monthly for cycles 4-7, and once every 8 weeks for up to 13 more cycles. The intermediate schedule consisted of treatment weekly in cycle 1 and every 8 weeks for up to 20 cycles. The short, intense schedule consisted of weekly treatment for 8 weeks (one cycle). Patients were followed for up to 4 years or until they progressed to multiple myeloma based on International Myeloma Working Group guidelines.
Over a median follow-up period of 15.8 months (range, 0 to 24 months), rates of complete response were 2% in the long treatment arm, 5% in the intermediate treatment arm, and 0% in the short treatment arm. Rates of at least very good partial response were 29%, 24%, and 15%, respectively. Overall response rates were 56%, 54%, and 38%, respectively. Median PFS was not reached in any arm, exceeding 24 months.
Treatment was generally well tolerated, said Dr. Hofmeister. The most common treatment-related adverse effects were fatigue, cough, upper respiratory tract infection, headache, and insomnia. Hypertension and hyperglycemia were the most common grade 3-4 treatment-emergent adverse events, affecting up to 5% of patients per arm. Fewer than 10% of patients in any arm developed treatment-emergent hematologic adverse events, and fewer than 5% developed grade 3-4 pneumonia or sepsis. There were three cases of a second primary malignancy, including one case of breast cancer and two cases of melanoma.
Rates of infusion-related reactions did not correlate with treatment duration. Grade 3-4 infusion-related reactions affected 0% to 3% of patients per arm. The sole death in this trial resulted from disease progression in a patient from the short treatment arm. “Taken together, efficacy and safety data support long dosing compared to intermediate and short dosing,” Dr. Hofmeister said.
The three arms were demographically similar. Patients tended to be white, in their late 50s to 60s, and to have ECOG scores of 0 with at least two risk factors for progression. About 70% had IgG disease and nearly half had less than 20% plasma cells in bone marrow.
Janssen, the maker of daratumumab, sponsored the trial. Dr. Hofmeister disclosed research funding from Janssen and research support, honoraria, and advisory relationships with Adaptive Biotechnologies, Thrasos, Celgene, Karyopharm, Takeda, and other pharmaceutical companies.
SOURCE: Hofmeister C et al, ASH 2017, Abstract 510.
ATLANTA – Daratumumab monotherapy led to durable partial responses among intermediate to high-risk patients with smoldering multiple myeloma, according to results from the phase II CENTAURUS trial.
Although less than 5% of patients had complete responses, 27% had at least a very good partial response to long-term therapy (up to 20 treatment cycles lasting 8 weeks each), Craig C. Hofmeister, MD, of the Ohio State University Comprehensive Cancer Center, Columbus, said at the annual meeting of the American Society of Hematology. The coprimary endpoint, median progression-free survival, exceeded 24 months in all dose cohorts, and was the longest when patients were treated longest.
Current guidelines recommend monitoring smoldering multiple myeloma every 3-6 months and treating only after patients progress. However, some experts pursue earlier treatment in the premalignant setting.
In CENTAURUS, 123 adults with smoldering multiple myeloma were randomly assigned to receive daratumumab (16 mg/kg IV) in 8-week cycles according to a long, intermediate, or short/intense schedule. The long schedule consisted of treatment weekly for cycle 1, every other week for cycles 2-3, monthly for cycles 4-7, and once every 8 weeks for up to 13 more cycles. The intermediate schedule consisted of treatment weekly in cycle 1 and every 8 weeks for up to 20 cycles. The short, intense schedule consisted of weekly treatment for 8 weeks (one cycle). Patients were followed for up to 4 years or until they progressed to multiple myeloma based on International Myeloma Working Group guidelines.
Over a median follow-up period of 15.8 months (range, 0 to 24 months), rates of complete response were 2% in the long treatment arm, 5% in the intermediate treatment arm, and 0% in the short treatment arm. Rates of at least very good partial response were 29%, 24%, and 15%, respectively. Overall response rates were 56%, 54%, and 38%, respectively. Median PFS was not reached in any arm, exceeding 24 months.
Treatment was generally well tolerated, said Dr. Hofmeister. The most common treatment-related adverse effects were fatigue, cough, upper respiratory tract infection, headache, and insomnia. Hypertension and hyperglycemia were the most common grade 3-4 treatment-emergent adverse events, affecting up to 5% of patients per arm. Fewer than 10% of patients in any arm developed treatment-emergent hematologic adverse events, and fewer than 5% developed grade 3-4 pneumonia or sepsis. There were three cases of a second primary malignancy, including one case of breast cancer and two cases of melanoma.
Rates of infusion-related reactions did not correlate with treatment duration. Grade 3-4 infusion-related reactions affected 0% to 3% of patients per arm. The sole death in this trial resulted from disease progression in a patient from the short treatment arm. “Taken together, efficacy and safety data support long dosing compared to intermediate and short dosing,” Dr. Hofmeister said.
The three arms were demographically similar. Patients tended to be white, in their late 50s to 60s, and to have ECOG scores of 0 with at least two risk factors for progression. About 70% had IgG disease and nearly half had less than 20% plasma cells in bone marrow.
Janssen, the maker of daratumumab, sponsored the trial. Dr. Hofmeister disclosed research funding from Janssen and research support, honoraria, and advisory relationships with Adaptive Biotechnologies, Thrasos, Celgene, Karyopharm, Takeda, and other pharmaceutical companies.
SOURCE: Hofmeister C et al, ASH 2017, Abstract 510.
REPORTING FROM ASH 2017
Key clinical point: Single-agent daratumumab therapy was active and its safety profile was acceptable in patients with smoldering multiple myeloma.
Major finding: Rates of at least very good partial response were 29%, 24%, and 15% among patients who received long, intermediate, and short/intense treatment schedules, respectively. Median progression-free survival exceeded 24 months in all three arms.
Data source: CENTAURUS, a phase II trial of 123 patients with smoldering multiple myeloma.
Disclosures: Janssen sponsored the trial. Dr. Hofmeister disclosed research funding from Janssen and research support, honoraria, and advisory relationships with Adaptive Biotechnologies, Thrasos, Celgene, Karyopharm, Takeda, and other pharmaceutical companies.
Source: Hofmeister C et al, ASH 2017, Abstract 510.
Study identifies predictors of acquired von Willebrand disease
ATLANTA – Waldenström macroglobulinemia can present as acquired von Willebrand disease (VWD), and when it does, the finding strongly correlates with high serum IgM levels and the presence of CXCR4 mutations, according to the results of a large, single-center retrospective study.
Further, successfully treating Waldenström macroglobulinemia often resolves acquired VWD, and the depth of treatment response predicts the degree of improvement, Jorge J. Castillo, MD, and his associates wrote in a poster presented at the annual meeting of the American Society of Hematology.
Acquired VWD is an uncommon, poorly understood presentation of Waldenström macroglobulinemia. Because affected patients require treatment, better characterizing this subgroup is important, the investigators noted.
At the Bing Center for Waldenström Macroglobulinemia at Dana-Farber Cancer Institute in Boston, the researchers retrospectively studied 320 individuals with newly diagnosed Waldenström macroglobulinemia and used logistic regression analysis to seek predictors of acquired VWD, which they evaluated by measuring levels of VW factor antigen, VW factor activity, and factor VIII. Levels under 30% were considered VWD and levels between 30% and 50% were considered low-level VWD.
In all, 49 individuals had acquired VWD while 271 patients did not. These two groups were similar in terms of age, sex, hemoglobin level, platelet count, and bone marrow involvement. However, 45% of patients with acquired VWD had serum IgM levels above 6,000 mg/dL versus 6% of patients without acquired VWD (P less than .001), and 47% of patients with acquired VWD had serum IgM levels between 3,000 and 5,999 versus 31% of patients without acquired VWD (P less than .001). Also, 77% of patients with acquired VWD tested positive for CXCR4 mutation versus 37% of patients without acquired VWD (P less than .001).
A significantly higher proportion of patients without acquired VWD had white blood cell concentrations above 6,000/mcL (29% vs. 50%; P = .006). This finding lost statistical significance in the logistic regression model, but all the other variables remained significantly associated. Serum IgM levels above 6,000 mg/dL conferred a 55-fold increase in the odds of having acquired VWD (95% confidence interval, 17-177; P less than .001), and serum IgM levels between 3,000 and 5,999 mg/dL led to an 11-fold increase in these odds (95% CI, 4-34). The presence of CXCR4 mutations was associated with a sixfold increased odds of acquired VWD (95% CI, 2-15). The P value for each of these three associations was at or below .001.
Therapy for Waldenström macroglobulinemia led to statistically significant increases in levels of factor VIII, VW factor antigen, and VW factor activity (P less than .001) and the median of each level improved by at least 35% after treatment. After treatment, 78% of patients with acquired VWD had levels of all three measures above 50% (versus 0% before treatment; P less than .001). Patients with acquired VWD with the best responses to treatment had about a 90% decrease in IgM levels, while those with a partial response had about a two-thirds decrease and patients with stable disease had about a 20% decrease. A linear regression model confirmed that depth of treatment response, based on change in IgM level, correlated with degree of improvement in VWD – that is, the extent of improvement in levels of VW factor antigen, VW factor activity, and factor VIII.
No external funding sources were reported. Dr. Castillo disclosed consulting ties and research funding from Pharmacyclics and Janssen, and research funding from Millenium and Abbvie.
SOURCE: Castillo J, et al. ASH 2017 Abstract 1088.
ATLANTA – Waldenström macroglobulinemia can present as acquired von Willebrand disease (VWD), and when it does, the finding strongly correlates with high serum IgM levels and the presence of CXCR4 mutations, according to the results of a large, single-center retrospective study.
Further, successfully treating Waldenström macroglobulinemia often resolves acquired VWD, and the depth of treatment response predicts the degree of improvement, Jorge J. Castillo, MD, and his associates wrote in a poster presented at the annual meeting of the American Society of Hematology.
Acquired VWD is an uncommon, poorly understood presentation of Waldenström macroglobulinemia. Because affected patients require treatment, better characterizing this subgroup is important, the investigators noted.
At the Bing Center for Waldenström Macroglobulinemia at Dana-Farber Cancer Institute in Boston, the researchers retrospectively studied 320 individuals with newly diagnosed Waldenström macroglobulinemia and used logistic regression analysis to seek predictors of acquired VWD, which they evaluated by measuring levels of VW factor antigen, VW factor activity, and factor VIII. Levels under 30% were considered VWD and levels between 30% and 50% were considered low-level VWD.
In all, 49 individuals had acquired VWD while 271 patients did not. These two groups were similar in terms of age, sex, hemoglobin level, platelet count, and bone marrow involvement. However, 45% of patients with acquired VWD had serum IgM levels above 6,000 mg/dL versus 6% of patients without acquired VWD (P less than .001), and 47% of patients with acquired VWD had serum IgM levels between 3,000 and 5,999 versus 31% of patients without acquired VWD (P less than .001). Also, 77% of patients with acquired VWD tested positive for CXCR4 mutation versus 37% of patients without acquired VWD (P less than .001).
A significantly higher proportion of patients without acquired VWD had white blood cell concentrations above 6,000/mcL (29% vs. 50%; P = .006). This finding lost statistical significance in the logistic regression model, but all the other variables remained significantly associated. Serum IgM levels above 6,000 mg/dL conferred a 55-fold increase in the odds of having acquired VWD (95% confidence interval, 17-177; P less than .001), and serum IgM levels between 3,000 and 5,999 mg/dL led to an 11-fold increase in these odds (95% CI, 4-34). The presence of CXCR4 mutations was associated with a sixfold increased odds of acquired VWD (95% CI, 2-15). The P value for each of these three associations was at or below .001.
Therapy for Waldenström macroglobulinemia led to statistically significant increases in levels of factor VIII, VW factor antigen, and VW factor activity (P less than .001) and the median of each level improved by at least 35% after treatment. After treatment, 78% of patients with acquired VWD had levels of all three measures above 50% (versus 0% before treatment; P less than .001). Patients with acquired VWD with the best responses to treatment had about a 90% decrease in IgM levels, while those with a partial response had about a two-thirds decrease and patients with stable disease had about a 20% decrease. A linear regression model confirmed that depth of treatment response, based on change in IgM level, correlated with degree of improvement in VWD – that is, the extent of improvement in levels of VW factor antigen, VW factor activity, and factor VIII.
No external funding sources were reported. Dr. Castillo disclosed consulting ties and research funding from Pharmacyclics and Janssen, and research funding from Millenium and Abbvie.
SOURCE: Castillo J, et al. ASH 2017 Abstract 1088.
ATLANTA – Waldenström macroglobulinemia can present as acquired von Willebrand disease (VWD), and when it does, the finding strongly correlates with high serum IgM levels and the presence of CXCR4 mutations, according to the results of a large, single-center retrospective study.
Further, successfully treating Waldenström macroglobulinemia often resolves acquired VWD, and the depth of treatment response predicts the degree of improvement, Jorge J. Castillo, MD, and his associates wrote in a poster presented at the annual meeting of the American Society of Hematology.
Acquired VWD is an uncommon, poorly understood presentation of Waldenström macroglobulinemia. Because affected patients require treatment, better characterizing this subgroup is important, the investigators noted.
At the Bing Center for Waldenström Macroglobulinemia at Dana-Farber Cancer Institute in Boston, the researchers retrospectively studied 320 individuals with newly diagnosed Waldenström macroglobulinemia and used logistic regression analysis to seek predictors of acquired VWD, which they evaluated by measuring levels of VW factor antigen, VW factor activity, and factor VIII. Levels under 30% were considered VWD and levels between 30% and 50% were considered low-level VWD.
In all, 49 individuals had acquired VWD while 271 patients did not. These two groups were similar in terms of age, sex, hemoglobin level, platelet count, and bone marrow involvement. However, 45% of patients with acquired VWD had serum IgM levels above 6,000 mg/dL versus 6% of patients without acquired VWD (P less than .001), and 47% of patients with acquired VWD had serum IgM levels between 3,000 and 5,999 versus 31% of patients without acquired VWD (P less than .001). Also, 77% of patients with acquired VWD tested positive for CXCR4 mutation versus 37% of patients without acquired VWD (P less than .001).
A significantly higher proportion of patients without acquired VWD had white blood cell concentrations above 6,000/mcL (29% vs. 50%; P = .006). This finding lost statistical significance in the logistic regression model, but all the other variables remained significantly associated. Serum IgM levels above 6,000 mg/dL conferred a 55-fold increase in the odds of having acquired VWD (95% confidence interval, 17-177; P less than .001), and serum IgM levels between 3,000 and 5,999 mg/dL led to an 11-fold increase in these odds (95% CI, 4-34). The presence of CXCR4 mutations was associated with a sixfold increased odds of acquired VWD (95% CI, 2-15). The P value for each of these three associations was at or below .001.
Therapy for Waldenström macroglobulinemia led to statistically significant increases in levels of factor VIII, VW factor antigen, and VW factor activity (P less than .001) and the median of each level improved by at least 35% after treatment. After treatment, 78% of patients with acquired VWD had levels of all three measures above 50% (versus 0% before treatment; P less than .001). Patients with acquired VWD with the best responses to treatment had about a 90% decrease in IgM levels, while those with a partial response had about a two-thirds decrease and patients with stable disease had about a 20% decrease. A linear regression model confirmed that depth of treatment response, based on change in IgM level, correlated with degree of improvement in VWD – that is, the extent of improvement in levels of VW factor antigen, VW factor activity, and factor VIII.
No external funding sources were reported. Dr. Castillo disclosed consulting ties and research funding from Pharmacyclics and Janssen, and research funding from Millenium and Abbvie.
SOURCE: Castillo J, et al. ASH 2017 Abstract 1088.
AT ASH 2017
Key clinical point: Successfully treating Waldenström macroglobulinemia often resolves acquired von Willebrand disease.
Major finding: Therapy for Waldenström macroglobulinemia led to statistically significant increases in levels of factor VIII, VW factor antigen, and VW factor activity (P less than .001) and the median of each level improved by at least 35% after treatment.
Data source: A single-center retrospective study of 320 patients with newly diagnosed Waldenström macroglobulinemia.
Disclosures: No external funding sources were reported. Dr. Castillo disclosed consultancy and research funding from Pharmacyclics and Janssen. He also disclosed research funding from Millenium and Abbvie.
Source: Castillo J, et al. ASH 2017 Abstract 1088.
bb2121 induces durable, deepening responses in MM patients
ATLANTA—Updated results from a phase 1 trial have shown that bb2121, a chimeric antigen receptor (CAR) T-cell product, can induce durable, deepening responses in patients with relapsed/refractory multiple myeloma (MM).
Responses continue to improve from very good partial responses to complete responses (CRs), even 15 months after infusion.
In 5 months, the CR rate increased from 27% to 56%, and ongoing responses have now surpassed 1 year.
The overall response rate (ORR) stands at 94%, and the median progression-free survival (PFS) has not been reached with a follow-up of 40 weeks.
bb2121 is a second-generation CAR T-cell product that targets the B-cell maturation antigen (BCMA).
BCMA is expressed nearly universally on MM cells, and its expression is largely restricted to plasma cells and some mature B cells, making it “an attractive target for immunotherapies,” said James N. Kochenderfer, MD, of the National Cancer Institute/National Institutes of Health in Bethesda, Maryland.
Dr Kochenderfer reported results from the phase 1 study of bb2121 (NCT02658929) at the 2017 ASH Annual Meeting (abstract 740*).
Study sponsors and collaborators were bluebird bio and Celgene Corporation. Dr Kochenderfer disclosed that he has multiple patents in the CAR field and has received research funding from bluebird bio and Kite Pharma.
Study design
Patients with relapsed or refractory MM who had 3 or more prior lines of therapy, including a proteasome inhibitor and immunomodulatory drug, or who were double refractory were eligible for the dose-escalation cohort of the study. They had to have measurable disease, 50% or more BCMA expression, and adequate bone marrow, renal, and hepatic function.
BCMA expression was not required for the dose-expansion cohort. For this cohort, patients must have received daratumumab and have been refractory to their last line of therapy.
The dose-escalation cohort was a standard 3 + 3 design and included CAR T-cell doses of 50 x 106, 150 x 106, 450 x 106, and 800 x 106.
Patients were screened, underwent leukapheresis, and waited for the manufacture of their CAR T cells. One centralized manufacturing site produced the T-cell products for the 9 US clinical study sites.
“We had a manufacturing success rate of 100%,” Dr Kochenderfer noted, and the manufacturing took 10 days.
Five days prior to bb2121 infusion, patients received lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2).
Patient characteristics
Investigators dosed 21 patients as of the data cut-off of October 2.
Their median age was 58 (range, 37–74), 62% were male, and they had a median time since diagnosis of 4 years.
All patients had an ECOG performance status of 0 or 1, and 43% had high-risk cytogenetics, defined as del17p, t(4;14), and t(14;16).
“One of the most impressive things about our study was how heavily pretreated the patients were,” Dr Kochenderfer noted. “These patients had a median of 7 prior lines of therapy, and 100% of the patients had a prior autologous stem cell transplant.”
All patients were exposed to bortezomib and lenalidomide, and 67% and 86%, respectively, were refractory to those agents. Patients were also exposed to carfilzomib (91%), pomalidomide (91%), and daratumumab (71%) and had varying degrees of refractoriness to those agents.
Safety
“In general, the treatment was very well tolerated,” Dr Kochenderfer said. “[It was] well tolerated compared with other T-cell products I’ve had experience with.”
The investigators observed no dose-limiting toxicities.
Cytokine release syndrome (CRS) of all grades occurred in 15 (71%) patients, and grade 3 or higher CRS occurred in 2 (10%) patients. The latter resolved within 24 hours.
Five (24%) patients experienced neurotoxicity, none grade 3 or higher.
Dr Kochenderfer described 1 case of delayed-onset, grade 4, reversible neurotoxicity that was associated with tumor lysis syndrome (TLS) and CRS.
The patient had no toxicity until day 10. By day 12, magnetic resonance imaging showed cerebral edema.
The patient was transferred to the intensive care unit and required intubation. She was treated with high-dose methylprednisolone and tocilizumab. She also received hemodialysis for TLS.
“By day 17, she dramatically improved,” Dr Kochenderfer said.
Her mental status cleared, TLS resolved, she was extubated, and she was doing much better, he reported.
On day 30, the patient was out of the intensive care unit.
“So the whole course was fairly brief,” Dr Kochenderfer said. “And, today, she’s doing well. She’s actually asymptomatic.”
Cytopenias—neutropenia, thrombocytopenia, and anemia—were primarily related to the lymphodepleting drugs, and patients recovered to grade 3 or lower by month 2 after the infusion.
Fourteen patients experienced 1 or more serious adverse events. Four had grade 1-2 CRS that required hospitalization per protocol, and 2 had pyrexia.
Five patients died, 3 due to disease progression, all who received treatment at the lowest dose.
Two patients treated at active doses were in CR when they died. One had a cardiac arrest, and the other had myelodysplastic syndrome following discontinuation.
Efficacy
In addition to the high ORR (94%) and CR rate (56%) in this study, 9 of 10 patients evaluated for minimal residual disease were negative.
The median time to first response was 1.02 months, and median time to best response was 3.74 months. The median time to CR was 3.84 months.
The median duration of response and PFS have not been reached. The PFS rate was 81% at 6 months and 71% at 9 months.
“We found that all the doses between 150 million and 450 million were effective,” Dr Kochenderfer noted. “We didn’t see a clear difference in efficacy between those doses, so we’ve chosen to use the 150 – 300 million dose range for the follow-up study.”
The investigators observed robust expansion of bb2121, which peaked in the first week after the infusion. Six of 13 patients had evident CAR T cells at 6 months. One patient has persistence over 12 months.
The investigators also observed a robust decrease in M protein and rapid clearance of serum-free light chains and serum BCMA. They noted that the activity of the CAR-positive T cells was not inhibited by high baseline serum BCMA.
Four patients progressed. The investigators analyzed the patients’ tumor burden, bb2121 dose, best response, time to progression, BCMA expression, grades of CRS, and bb2121 persistence. And progression was independent of these factors.
“So we can’t pick out a very good factor of why they progressed,” Dr Kochenderfer said.
However, he noted that the patients are eligible for re-treatment.
Investigators have opened a global trial of bb2121 (NCT03361748) given at doses ranging from 150 – 300 million CAR T cells.
The US Food and Drug Administration and the European Medicines Agency recently fast-tracked bb2121. ![]()
*Data presented differ from the abstract.
ATLANTA—Updated results from a phase 1 trial have shown that bb2121, a chimeric antigen receptor (CAR) T-cell product, can induce durable, deepening responses in patients with relapsed/refractory multiple myeloma (MM).
Responses continue to improve from very good partial responses to complete responses (CRs), even 15 months after infusion.
In 5 months, the CR rate increased from 27% to 56%, and ongoing responses have now surpassed 1 year.
The overall response rate (ORR) stands at 94%, and the median progression-free survival (PFS) has not been reached with a follow-up of 40 weeks.
bb2121 is a second-generation CAR T-cell product that targets the B-cell maturation antigen (BCMA).
BCMA is expressed nearly universally on MM cells, and its expression is largely restricted to plasma cells and some mature B cells, making it “an attractive target for immunotherapies,” said James N. Kochenderfer, MD, of the National Cancer Institute/National Institutes of Health in Bethesda, Maryland.
Dr Kochenderfer reported results from the phase 1 study of bb2121 (NCT02658929) at the 2017 ASH Annual Meeting (abstract 740*).
Study sponsors and collaborators were bluebird bio and Celgene Corporation. Dr Kochenderfer disclosed that he has multiple patents in the CAR field and has received research funding from bluebird bio and Kite Pharma.
Study design
Patients with relapsed or refractory MM who had 3 or more prior lines of therapy, including a proteasome inhibitor and immunomodulatory drug, or who were double refractory were eligible for the dose-escalation cohort of the study. They had to have measurable disease, 50% or more BCMA expression, and adequate bone marrow, renal, and hepatic function.
BCMA expression was not required for the dose-expansion cohort. For this cohort, patients must have received daratumumab and have been refractory to their last line of therapy.
The dose-escalation cohort was a standard 3 + 3 design and included CAR T-cell doses of 50 x 106, 150 x 106, 450 x 106, and 800 x 106.
Patients were screened, underwent leukapheresis, and waited for the manufacture of their CAR T cells. One centralized manufacturing site produced the T-cell products for the 9 US clinical study sites.
“We had a manufacturing success rate of 100%,” Dr Kochenderfer noted, and the manufacturing took 10 days.
Five days prior to bb2121 infusion, patients received lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2).
Patient characteristics
Investigators dosed 21 patients as of the data cut-off of October 2.
Their median age was 58 (range, 37–74), 62% were male, and they had a median time since diagnosis of 4 years.
All patients had an ECOG performance status of 0 or 1, and 43% had high-risk cytogenetics, defined as del17p, t(4;14), and t(14;16).
“One of the most impressive things about our study was how heavily pretreated the patients were,” Dr Kochenderfer noted. “These patients had a median of 7 prior lines of therapy, and 100% of the patients had a prior autologous stem cell transplant.”
All patients were exposed to bortezomib and lenalidomide, and 67% and 86%, respectively, were refractory to those agents. Patients were also exposed to carfilzomib (91%), pomalidomide (91%), and daratumumab (71%) and had varying degrees of refractoriness to those agents.
Safety
“In general, the treatment was very well tolerated,” Dr Kochenderfer said. “[It was] well tolerated compared with other T-cell products I’ve had experience with.”
The investigators observed no dose-limiting toxicities.
Cytokine release syndrome (CRS) of all grades occurred in 15 (71%) patients, and grade 3 or higher CRS occurred in 2 (10%) patients. The latter resolved within 24 hours.
Five (24%) patients experienced neurotoxicity, none grade 3 or higher.
Dr Kochenderfer described 1 case of delayed-onset, grade 4, reversible neurotoxicity that was associated with tumor lysis syndrome (TLS) and CRS.
The patient had no toxicity until day 10. By day 12, magnetic resonance imaging showed cerebral edema.
The patient was transferred to the intensive care unit and required intubation. She was treated with high-dose methylprednisolone and tocilizumab. She also received hemodialysis for TLS.
“By day 17, she dramatically improved,” Dr Kochenderfer said.
Her mental status cleared, TLS resolved, she was extubated, and she was doing much better, he reported.
On day 30, the patient was out of the intensive care unit.
“So the whole course was fairly brief,” Dr Kochenderfer said. “And, today, she’s doing well. She’s actually asymptomatic.”
Cytopenias—neutropenia, thrombocytopenia, and anemia—were primarily related to the lymphodepleting drugs, and patients recovered to grade 3 or lower by month 2 after the infusion.
Fourteen patients experienced 1 or more serious adverse events. Four had grade 1-2 CRS that required hospitalization per protocol, and 2 had pyrexia.
Five patients died, 3 due to disease progression, all who received treatment at the lowest dose.
Two patients treated at active doses were in CR when they died. One had a cardiac arrest, and the other had myelodysplastic syndrome following discontinuation.
Efficacy
In addition to the high ORR (94%) and CR rate (56%) in this study, 9 of 10 patients evaluated for minimal residual disease were negative.
The median time to first response was 1.02 months, and median time to best response was 3.74 months. The median time to CR was 3.84 months.
The median duration of response and PFS have not been reached. The PFS rate was 81% at 6 months and 71% at 9 months.
“We found that all the doses between 150 million and 450 million were effective,” Dr Kochenderfer noted. “We didn’t see a clear difference in efficacy between those doses, so we’ve chosen to use the 150 – 300 million dose range for the follow-up study.”
The investigators observed robust expansion of bb2121, which peaked in the first week after the infusion. Six of 13 patients had evident CAR T cells at 6 months. One patient has persistence over 12 months.
The investigators also observed a robust decrease in M protein and rapid clearance of serum-free light chains and serum BCMA. They noted that the activity of the CAR-positive T cells was not inhibited by high baseline serum BCMA.
Four patients progressed. The investigators analyzed the patients’ tumor burden, bb2121 dose, best response, time to progression, BCMA expression, grades of CRS, and bb2121 persistence. And progression was independent of these factors.
“So we can’t pick out a very good factor of why they progressed,” Dr Kochenderfer said.
However, he noted that the patients are eligible for re-treatment.
Investigators have opened a global trial of bb2121 (NCT03361748) given at doses ranging from 150 – 300 million CAR T cells.
The US Food and Drug Administration and the European Medicines Agency recently fast-tracked bb2121. ![]()
*Data presented differ from the abstract.
ATLANTA—Updated results from a phase 1 trial have shown that bb2121, a chimeric antigen receptor (CAR) T-cell product, can induce durable, deepening responses in patients with relapsed/refractory multiple myeloma (MM).
Responses continue to improve from very good partial responses to complete responses (CRs), even 15 months after infusion.
In 5 months, the CR rate increased from 27% to 56%, and ongoing responses have now surpassed 1 year.
The overall response rate (ORR) stands at 94%, and the median progression-free survival (PFS) has not been reached with a follow-up of 40 weeks.
bb2121 is a second-generation CAR T-cell product that targets the B-cell maturation antigen (BCMA).
BCMA is expressed nearly universally on MM cells, and its expression is largely restricted to plasma cells and some mature B cells, making it “an attractive target for immunotherapies,” said James N. Kochenderfer, MD, of the National Cancer Institute/National Institutes of Health in Bethesda, Maryland.
Dr Kochenderfer reported results from the phase 1 study of bb2121 (NCT02658929) at the 2017 ASH Annual Meeting (abstract 740*).
Study sponsors and collaborators were bluebird bio and Celgene Corporation. Dr Kochenderfer disclosed that he has multiple patents in the CAR field and has received research funding from bluebird bio and Kite Pharma.
Study design
Patients with relapsed or refractory MM who had 3 or more prior lines of therapy, including a proteasome inhibitor and immunomodulatory drug, or who were double refractory were eligible for the dose-escalation cohort of the study. They had to have measurable disease, 50% or more BCMA expression, and adequate bone marrow, renal, and hepatic function.
BCMA expression was not required for the dose-expansion cohort. For this cohort, patients must have received daratumumab and have been refractory to their last line of therapy.
The dose-escalation cohort was a standard 3 + 3 design and included CAR T-cell doses of 50 x 106, 150 x 106, 450 x 106, and 800 x 106.
Patients were screened, underwent leukapheresis, and waited for the manufacture of their CAR T cells. One centralized manufacturing site produced the T-cell products for the 9 US clinical study sites.
“We had a manufacturing success rate of 100%,” Dr Kochenderfer noted, and the manufacturing took 10 days.
Five days prior to bb2121 infusion, patients received lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2).
Patient characteristics
Investigators dosed 21 patients as of the data cut-off of October 2.
Their median age was 58 (range, 37–74), 62% were male, and they had a median time since diagnosis of 4 years.
All patients had an ECOG performance status of 0 or 1, and 43% had high-risk cytogenetics, defined as del17p, t(4;14), and t(14;16).
“One of the most impressive things about our study was how heavily pretreated the patients were,” Dr Kochenderfer noted. “These patients had a median of 7 prior lines of therapy, and 100% of the patients had a prior autologous stem cell transplant.”
All patients were exposed to bortezomib and lenalidomide, and 67% and 86%, respectively, were refractory to those agents. Patients were also exposed to carfilzomib (91%), pomalidomide (91%), and daratumumab (71%) and had varying degrees of refractoriness to those agents.
Safety
“In general, the treatment was very well tolerated,” Dr Kochenderfer said. “[It was] well tolerated compared with other T-cell products I’ve had experience with.”
The investigators observed no dose-limiting toxicities.
Cytokine release syndrome (CRS) of all grades occurred in 15 (71%) patients, and grade 3 or higher CRS occurred in 2 (10%) patients. The latter resolved within 24 hours.
Five (24%) patients experienced neurotoxicity, none grade 3 or higher.
Dr Kochenderfer described 1 case of delayed-onset, grade 4, reversible neurotoxicity that was associated with tumor lysis syndrome (TLS) and CRS.
The patient had no toxicity until day 10. By day 12, magnetic resonance imaging showed cerebral edema.
The patient was transferred to the intensive care unit and required intubation. She was treated with high-dose methylprednisolone and tocilizumab. She also received hemodialysis for TLS.
“By day 17, she dramatically improved,” Dr Kochenderfer said.
Her mental status cleared, TLS resolved, she was extubated, and she was doing much better, he reported.
On day 30, the patient was out of the intensive care unit.
“So the whole course was fairly brief,” Dr Kochenderfer said. “And, today, she’s doing well. She’s actually asymptomatic.”
Cytopenias—neutropenia, thrombocytopenia, and anemia—were primarily related to the lymphodepleting drugs, and patients recovered to grade 3 or lower by month 2 after the infusion.
Fourteen patients experienced 1 or more serious adverse events. Four had grade 1-2 CRS that required hospitalization per protocol, and 2 had pyrexia.
Five patients died, 3 due to disease progression, all who received treatment at the lowest dose.
Two patients treated at active doses were in CR when they died. One had a cardiac arrest, and the other had myelodysplastic syndrome following discontinuation.
Efficacy
In addition to the high ORR (94%) and CR rate (56%) in this study, 9 of 10 patients evaluated for minimal residual disease were negative.
The median time to first response was 1.02 months, and median time to best response was 3.74 months. The median time to CR was 3.84 months.
The median duration of response and PFS have not been reached. The PFS rate was 81% at 6 months and 71% at 9 months.
“We found that all the doses between 150 million and 450 million were effective,” Dr Kochenderfer noted. “We didn’t see a clear difference in efficacy between those doses, so we’ve chosen to use the 150 – 300 million dose range for the follow-up study.”
The investigators observed robust expansion of bb2121, which peaked in the first week after the infusion. Six of 13 patients had evident CAR T cells at 6 months. One patient has persistence over 12 months.
The investigators also observed a robust decrease in M protein and rapid clearance of serum-free light chains and serum BCMA. They noted that the activity of the CAR-positive T cells was not inhibited by high baseline serum BCMA.
Four patients progressed. The investigators analyzed the patients’ tumor burden, bb2121 dose, best response, time to progression, BCMA expression, grades of CRS, and bb2121 persistence. And progression was independent of these factors.
“So we can’t pick out a very good factor of why they progressed,” Dr Kochenderfer said.
However, he noted that the patients are eligible for re-treatment.
Investigators have opened a global trial of bb2121 (NCT03361748) given at doses ranging from 150 – 300 million CAR T cells.
The US Food and Drug Administration and the European Medicines Agency recently fast-tracked bb2121. ![]()
*Data presented differ from the abstract.
Chemo-free combo should be option for rel/ref CLL, doc says
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
Drug allows for treatment-free periods in PV
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
Risk stratification may be possible with JCAR017
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.