User login
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
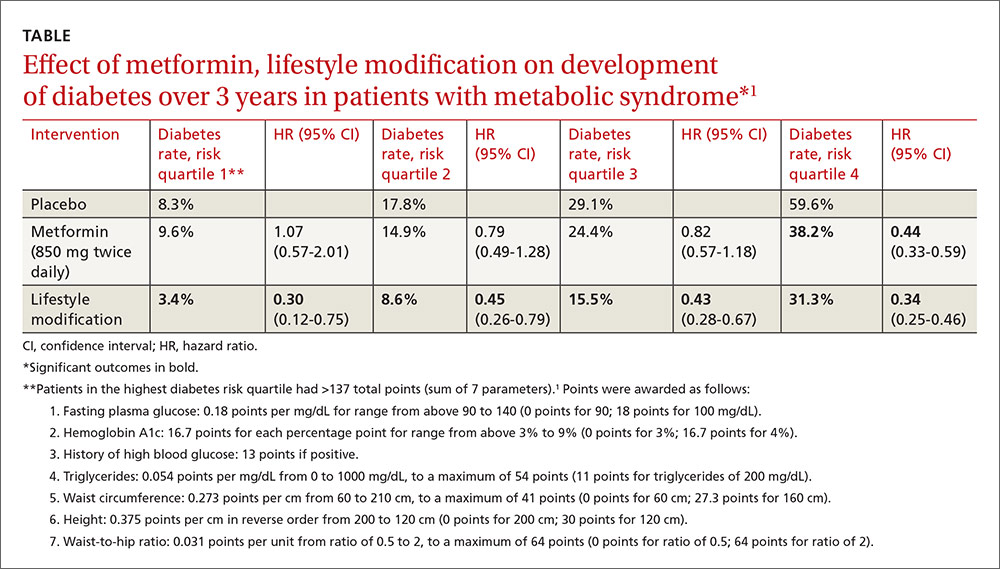
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).
Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).
Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
EVIDENCE-BASED ANSWER:
Patients at highest risk for progression to diabetes benefit from metformin.
In patients with metabolic syndrome who are in the highest-risk quartile for progression to diabetes (predicted mean 3-year risk, 60%), metformin, 850 mg twice daily, reduces the absolute risk by about 20% over a 3-year period. Metformin doesn’t reduce the incidence in patients at lower risk of progression (strength of recommendation [SOR]: C, post-hoc analysis of a randomized controlled trial [RCT]).
Intensive lifestyle modification reduces absolute risk in all patients proportionate to risk quartile (from 5% reduction for the lowest quartile to 28% for the highest). Over a 10-year period, intensive lifestyle modification reduces the absolute risk of diabetes by 34% and metformin reduces the risk by 18% for all patients at increased risk (considered as a group)—that is, not separated by risk quartile (SOR: A, large prospective RCTs).
Lower doses or shorter courses of metformin reduce fasting plasma glucose (SOR: C, RCTs with laboratory outcomes) and may reduce the risk of developing diabetes by a smaller amount (SOR: C, flawed RCT).
EVIDENCE SUMMARY
A post-hoc analysis of a prospective RCT (the Diabetes Prevention Program) comprising 3081 patients with impaired glucose metabolism who received metformin, a lifestyle modification program, or no intervention (placebo) found that metformin reduced the risk of developing diabetes only for patients in the highest risk quartile over 2.8 years. Lifestyle modification reduced diabetes risk in all patients.1
Investigators stratified patients who met National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for metabolic syndrome into risk quartiles for progression to diabetes using a model they developed based on 7 parameters: fasting plasma glucose, hemoglobin A1c, history of high blood glucose, waist:hip ratio, waist circumference, triglycerides, and height (TABLE1). The model reasonably fit outcomes—the percentage of patients in each quartile who developed diabetes—with an area under the receiver operator characteristic curve of 0.73 (a measure of diagnostic accuracy where 1 is a perfect predictor and 0.5 is random).
Lifestyle modification reduced risk in all quartiles with progressively greater effect as risk increased (lowest risk quartile: ARR=4.9%, 3-year NNT=20.4; highest risk quartile: ARR=28.3%; 3-year NNT=3.5).
There were 2 key weaknesses of the risk model: It wasn’t validated in a separate population and the true incidence of diabetes among patients taking placebo was higher than predicted. The investigators compared their risk prediction model results with the Framingham Risk Score (FRS) for diabetes and found that they correlated well, although the FRS results were consistently about 6% (absolute) higher when corrected for duration. (The FRS calculator is available online at www.framinghamheartstudy.org/risk-functions/diabetes/.)
Lifestyle change reduces diabetes risk more than metformin
The original Diabetes Prevention Program found that intensive lifestyle intervention and metformin reduced the number of diabetes cases over 2.8 years among 3234 patients at risk for developing diabetes.2
Compared with no intervention, fewer patients developed diabetes with either metformin or lifestyle improvement, although lifestyle change had the larger effect (no intervention: 11 cases per 100 person-years; metformin: 7.8 cases; 95% confidence interval [CI], 6.8-8.8; ARR=3.2% per year vs no intervention; lifestyle improvements: 4.8 cases; 95% CI, 4.1-5.7; ARR=6.2% per year vs no intervention).
The effect of metformin and lifestyle change persists at 10 years
A 10-year follow-up study to the Diabetes Prevention Program found that, compared with no intervention, both metformin and lifestyle interventions continued to be associated with a lower incidence of diabetes (no intervention: 7.8 cases per 100 person-years; 95% CI, 4.8-6.5; metformin: 6.4 cases; 95% CI, 4.2-5.7; ARR=1.4% per year; lifestyle interventions: 5.3 cases; 95% CI, 5.1-6.8; ARR=2.5% per year).3
Researchers originally randomized 3234 patients with body mass index ≥24 kg/m2, fasting blood sugar 95 to 125 mg/dL, and 2-hour post 75-gm glucose value of 149 to 199 mg/dL to 3 groups: intensive lifestyle modification (weight loss goal of 7%, 150 minutes a week of exercise), metformin (850 mg twice daily), and no intervention. After the 2.8-year follow-up period, 2766 patients continued for another 5.7 years of follow-up. Investigators offered group lifestyle counseling to all patients and continued metformin at the same dose in the second group.
Earlier study shows an effect for metformin, but with a caveat
An earlier RCT found that metformin reduced the risk of developing diabetes in patients with metabolic syndrome.4 Investigators randomized 70 patients to metformin (250 mg 3 times daily) or placebo for a year. Fewer patients developed diabetes with metformin (3% vs 16.2%, P=.011; NNT=7.6) and more had a normal glucose tolerance test result (84.9% vs 51.4%, P=.011; NNT=3). However, by current American Diabetes Association criteria, half of the subjects had early diabetes at baseline.
Metformin lowers fasting blood sugar, but may not reverse metabolic syndrome
A post-hoc analysis of another RCT found that metformin reduced fasting plasma glucose (FPG) levels in patients with upper-body obesity and metabolic syndrome (by 1999 World Health Organization criteria but not NCEP ATP III criteria).5
Investigators randomized 457 patients to metformin 850 mg once daily or placebo and followed them for a year. FPG levels decreased with metformin but increased with placebo (reduction FPG 5.9 mg/dL vs increase FPG 12.3 mg/dL; P<.04). The investigators didn’t report whether any patients developed diabetes.
However, another RCT (155 patients) that compared metformin 850 mg twice daily with placebo in subjects with metabolic syndrome but without diabetes found greater normalization of FPG (5% vs 0%; P=.005), but no reversal of metabolic syndrome or change in Framingham 10-year risk score after 12 weeks.6
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
1. Sussman JB, Kent DM, Nelson JP, et al. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454.
2. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Outcomes Study. Lancet. 2009:374:1677-1686.
4. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabetes Med. 1999;16:477-481.
5. Fontbonne A, Diouf I, Baccara-Dinet M, et al. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391.
6. Nieuwdorp M, Stroes ESG, Kastelein JJP. Normalization of metabolic syndrome using fenofibrate, metformin or their combination. Diabetes Obesity Metab. 2007;9:869-878.
Evidence-based answers from the Family Physicians Inquiries Network