User login
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
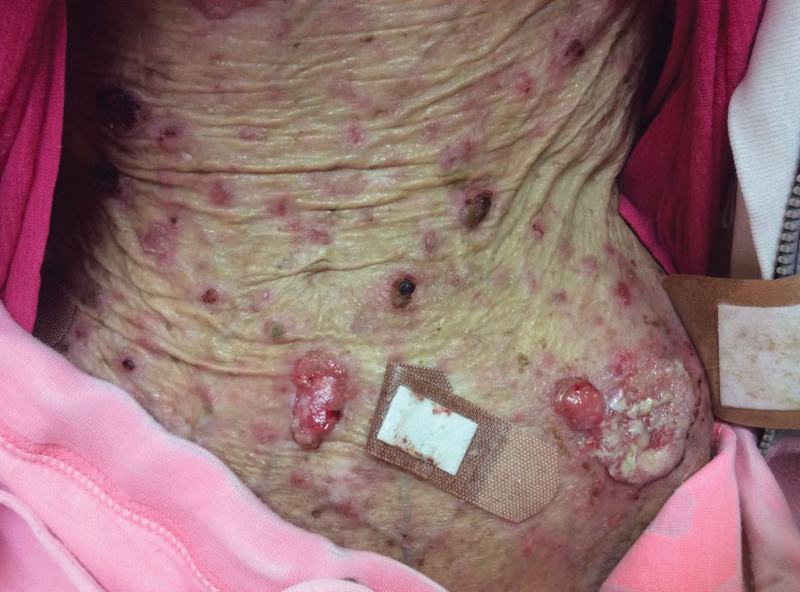
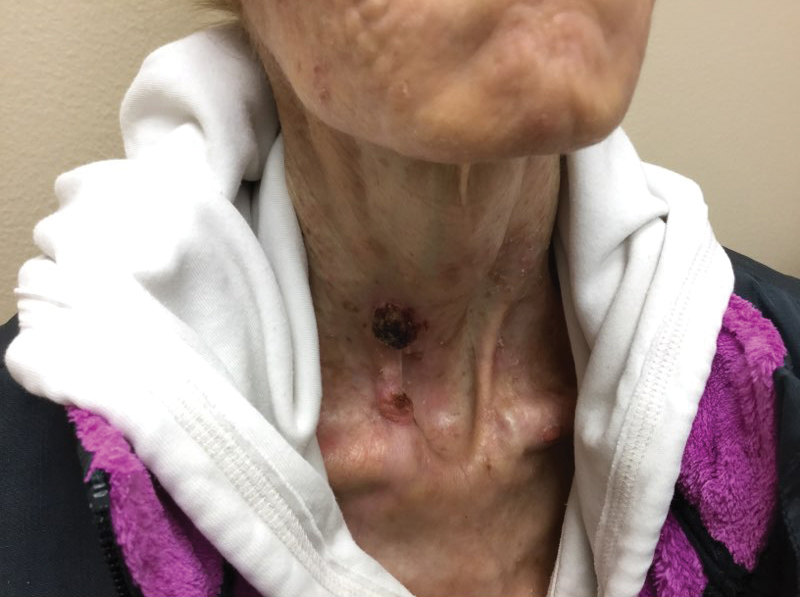
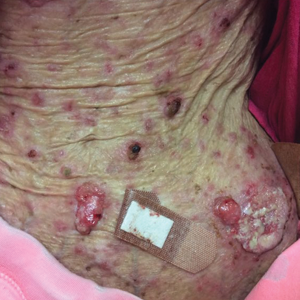
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
Practice Points
- Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.
- Comorbidities related to TANS syndrome make it challenging to effectively treat cutaneous squamous cell carcinoma.