User login
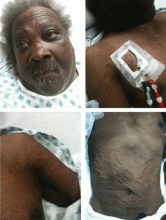
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
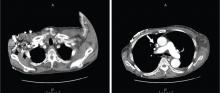
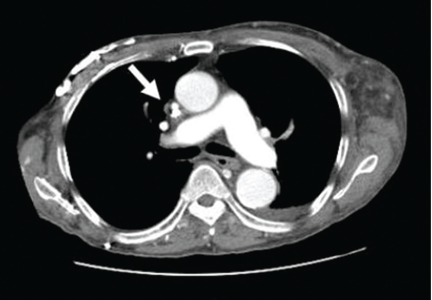
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.