User login
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
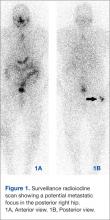
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.