User login
The following case report describes the use of dexmedetomidine as the primary sedative for an awake endotracheal intubation, as an adjuvant for general anesthesia, and for postoperative sedation for mechanical ventilation. This case illustrates problems that attracted the attention of federal institutions, specifically the management of difficult airways (with and without anatomic distortion), obesity, and obstructive sleep apnea (OSA). As such, it is of potential interest not only to anesthesiologists, but also other health care providers in the VA, especially those who might practice in intensive care settings.
Dexmedetomidine has useful pharmacologic properties that have potential use in a wide variety of clinical scenarios. Dexmedetomidine is currently indicated for sedation in nonintubated patients before and during surgical and other procedures and in intubated and mechanically ventilated patients during treatment in an intensive care setting.
Large neck masses can produce numerous problems that complicate the anesthetic management in the intraoperative and immediate postoperative arenas. The adjuvant use of dexmedetomidine, an alpha-2 agonist that has useful properties for both the anesthetic and intensive care situations, will be discussed. The problems involved with the management and resection of large neck masses include tracheal deviation, tracheal compression, airway edema, distorted anatomy, difficult mask ventilation, difficult intubation, postoperative recurrent laryngeal nerve dysfunction, and difficult exposure for tracheostomy.
Case Report
A 46-year-old man was referred for removal of a large thyroid mass. His past medical history included hypertension, obesity, and type 2 diabetes mellitus. Clinically, the patient seemed to be at risk for OSA, but he had not received a formal diagnosis. The patient met many of the criteria for screening OSA that are listed for a STOP-Bang Questionnaire.1 He was clinically and serologically euthyroid. Neck ultrasound revealed a very large thyroid mass with cystic and solid lesions throughout. Other than hoarseness, the patient reported no compressive symptoms, such as dysphagia or airway compromise. He was maintained on metoprolol, fosinopril, a thiazide for hypertension, and metformin and insulin for diabetes. A physical examination was remarkable for a Mallampati IV airway classification, a 61-cm neck circumference, 177 cm height, 142 kg weight, and a body mass index of 45. These preoperative assessments were predictive of a high probability of very difficult mask ventilation and intubation after the induction of a general anesthetic, or in any other situation requiring tracheal intubation, such as respiratory failure in the postoperative period.
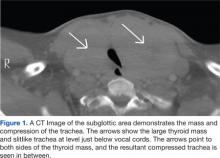
Preoperative laboratory studies, chest radiograph, and electrocardiogram (ECG) were unremarkable. Computed tomography (CT) imaging of the neck revealed marked enlargement of the thyroid, which had a multinodular, heterogeneous appearance with scattered calcifications. The left lobe of the thyroid measured 13.0 cm craniocaudal by 9.47 cm transverse by 6.8 cm anteroposterior. The right lobe of the thyroid measured 12.0 cm craniocaudal by 7.6 cm transverse by 7.0 cm anteroposterior (Figure 1).
The first concern for this patient was a secure airway, which potentially could have been very difficult to procure with a standard IV induction of anesthesia followed by a direct laryngoscopy. This was further constrained by the surgical requirement that the patient be intubated with an electromyography (EMG) endotracheal tube for monitoring of the recurrent laryngeal nerves, as thyroid surgery carries the risk of injury to these nerves. The type of tube that was used had a larger diameter than that of a standard endotracheal tube (the EMG tube measured 10.2 mm outside diameter vs 9.6 mm outside diameter for a standard tube) but was also far more rigid, precluding nasal intubation and making navigation of the tip around corners and obstructions more difficult. A final laryngoscopy was also needed for confirmation of optimal electrode placement at the vocal cord level (Figure 2).
The anesthetic plan was to secure the airway with an awake oral fiberoptic intubation under sedation and topical local anesthetic to avoid the hypoxemia that would ensue if the patient lost spontaneous respiration. The patient was brought without preoperative sedation to the operating room, standard monitors (eg, ECG, noninvasive blood pressure, pulse oximetry) were applied and IV access was obtained. Blood pressure, heart rate, and oxygen saturation were within normal limits. He was placed on oxygen 2 L/min by nasal cannula and given a 1 μg/kg loading dose of dexmedetomidine over 10 minutes and thereafter maintained on a 0.4 μg/kg/h maintenance infusion during the entire airway intubation sequence. A topical anesthesia of 4% lidocaine spray was applied to the upper airway, and a transtracheal injection was performed with 2 mL of 4% lidocaine. The patient’s anatomy precluded the use of superior laryngeal nerve blocks. During the dexmedetomidine loading, he was given 1 mg midazolam and 100 μg fentanyl IV incrementally. No significant hemodynamic or respiratory changes occurred with this sedation regimen.
An attempt to place an oral intubation bite block failed, because the stiff EMG tube proved too difficult to pass through it. Therefore, the EMG tube and rolled gauze pads placed between the upper and lower teeth were used to protect the fiberoptic bronchoscope while it was guided past the base of the tongue. As was noted in the CT scan, the airway was deviated slightly to the left, and this information was useful for guiding the fiberscope. The location of the epiglottis was fairly difficult to ascertain due to redundant tissue in the hypopharyngeal area but was ultimately visible through the fiberscope.
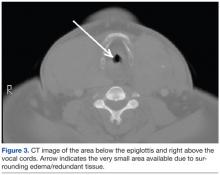
The vocal cords were not visible, possibly due to the significant amount of airway edema and/or redundant tissue between the epiglottis and the vocal cords: Only the space beneath the epiglottis could be seen via the fiberscope. Passing the bronchoscope through the larynx also was problematic due to what may be described as altered spatial/angular relationships and due to the supraglottic edema/tissue leaving little room for the tip of the bronchoscope to be maneuvered. Figure 3 shows a CT scan image of the supraglottic area.
It took 45 minutes and multiple attempts to pass the bronchoscope into the trachea. The dexmedetomidine infusion was continued throughout this entire sequence. The patient tolerated this manipulation with little difficulty, despite the multiple airway maneuvers, and his hemodynamic and respiratory status remained clinically stable. Oxygen saturation was 95% to 100% during this sequence and the patient did not show evidence of significant upper airway collapse, desaturation, or apnea, which are sometimes encountered during sedation for airway manipulation.
The patient’s hemodynamic status remained near baseline values throughout the airway manipulation. The patient never lost his ability to cooperate. After manipulation of the fiberscope into the trachea, the tracheal rings and carina were visualized, and the tube was advanced over the scope. Minimal to mild coughing occurred once the tube passed through the vocal cords. The tube position in the trachea was verified with end-tidal CO2 and bronchoscopy and then the induction of anesthesia with propofol was completed. A laryngoscopy using a videolaryngoscope confirmed proper EMG electrode placement. Large-bore IV access and an arterial line were then secured.
The operation lasted about 15 hours. Maintenance of anesthesia was accomplished with the use of the volatile anesthetic desflurane, titrated to patient response to the surgical procedure. Additionally, 550 μg of IV fentanyl was used intermittently during the operation. Dexmedetomidine was infused at a rate of 0.2 to 0.4 μg/kg/h during the anesthetic, titrated to hemodynamic response. All hemodynamic parameters remained stable and within 20% of preoperative levels during the procedure. The blood loss during the procedure was minimal (< 100 mL), and acceptable readings from the EMG tube were confirmed throughout the surgical procedure.
The 686-gram thyroid mass was confirmed to be a multinodular goiter. Due to the difficulty with intubation, the length of the surgical procedure, and the likelihood of airway difficulties from edema possibly requiring reintubation, the patient was left intubated and mechanically ventilated overnight and sedated with a dexmedetomidine infusion of 0.3 μg/kg/h and propofol 35 mL/h. No further medications were required. He tolerated the ventilator without fighting, straining, coughing, or hypertensive responses and remained cooperative when aroused. He was successfully extubated the following day. Afterward, the patient maintained his airway and had only a mild right vocal cord paresis complicating his surgical management.
Discussion
The critical issues associated with this successful endotracheal intubation included the patient’s obesity, thyroid mass size, and deviation/compression of the trachea. Were this patient morbidly obese only, airway management would still be problematic; this was exacerbated by the concurrent pathologies. Dexmedetomidine possesses several advantageous properties for the perioperative period and was chosen as sedation for the awake intubation due to its sedative-analgesic effects, opioid sparing effects, lack of respiratory depression, maintenance of patient cooperation, and antisialagogue effect.2-5 Dexmedetomidine has previously been shown to be useful for awake intubation of difficult airway cases.4,6,7 Importantly, the dexmedetomidine sedation seemed to blunt the hypertensive responses often seen during airway maneuvers.8 It was also chosen as an intraoperative adjunct due to the above-noted opioid-sparing effects, given the importance of minimizing perioperative opioids needed for this morbidly obese individual with airway compromise.
In the literature, dexmedetomidine has been shown to greatly reduce the need for opioids, both intraoperatively and immediately postoperatively in many citations, for example, in postoperative mechanically ventilated coronary artery bypass graft patients.2,9 Opioid usage reduction is especially needed for the morbidly obese who are at increased risk of OSA and the attendant increased sensitivity to the respiratory depressant effects of narcotics. Postoperative opioids are being debated in the literature as potentially being a risk factor for cancer recurrence due to the effects on the immune system.5,10
Although the pathology report was benign for this patient, it was thought that prior to the surgery a reduction in opioid usage was important because he may have had a thyroid carcinoma in addition to the other respiratory considerations. Additionally, it was desired to decrease the amount of volatile anesthetic agents needed for this patient, because the surgical procedure was anticipated to be quite prolonged (it lasted 15 hours).
Conclusions
Recent research showed that a dexmedetomidine infusion combined with a low-dosage midazolam was superior to a higher dosage midazolam regimen for awake fiberoptic intubation in terms of stability, comfort, cooperation, and patient satisfaction.11 This is an example of the utility of dexmedetomidine. It is often insufficient when used alone, but as an adjunct will markedly reduce the dosage of other sedatives needed to achieve the desired Ramsey sedation scores and/or clinical benefit. Additionally, dexmedetomidine has been shown to facilitate weaning patients in a case series (who had previously failed weaning) from mechanical ventilation in surgical intensive care settings.12
The use of dexmedetomidine facilitated awake intubation for this patient and was helpful for postoperative sedation. The authors believe that dexmedetomidine has potential benefits in all phases of surgery and is a potentially valuable addition to the anesthesiologist’s and intensivist’s armamentarium.
Acknowledgements
This work was conducted at and supported by the G.V. (Sonny) Montgomery VA Medical Center in Jackson, Mississippi.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Hathaway B, Johnson JT. Safety of uvulopalatopharyngoplasty as outpatient surgery. Otolaryngol Head Neck Surg. 2006;134(4):542-544.
2. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cen). 2001;14(1):13-21.
3. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53(7):646-652.
4. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382-394.
5. Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136-1142.
6. Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19(4):141-144.
7. Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: A report of three cases. J Clin Anesth. 2004;16(2):124-126.
8. Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7(1):43-52.
9. Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576-584.
10. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109(2):180-187.
11. Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22(1):35-40.
12. Siobal MS, Kallet RH, Kivett VA, Tang JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir Care. 2006;51(5):492-496.
The following case report describes the use of dexmedetomidine as the primary sedative for an awake endotracheal intubation, as an adjuvant for general anesthesia, and for postoperative sedation for mechanical ventilation. This case illustrates problems that attracted the attention of federal institutions, specifically the management of difficult airways (with and without anatomic distortion), obesity, and obstructive sleep apnea (OSA). As such, it is of potential interest not only to anesthesiologists, but also other health care providers in the VA, especially those who might practice in intensive care settings.
Dexmedetomidine has useful pharmacologic properties that have potential use in a wide variety of clinical scenarios. Dexmedetomidine is currently indicated for sedation in nonintubated patients before and during surgical and other procedures and in intubated and mechanically ventilated patients during treatment in an intensive care setting.
Large neck masses can produce numerous problems that complicate the anesthetic management in the intraoperative and immediate postoperative arenas. The adjuvant use of dexmedetomidine, an alpha-2 agonist that has useful properties for both the anesthetic and intensive care situations, will be discussed. The problems involved with the management and resection of large neck masses include tracheal deviation, tracheal compression, airway edema, distorted anatomy, difficult mask ventilation, difficult intubation, postoperative recurrent laryngeal nerve dysfunction, and difficult exposure for tracheostomy.
Case Report
A 46-year-old man was referred for removal of a large thyroid mass. His past medical history included hypertension, obesity, and type 2 diabetes mellitus. Clinically, the patient seemed to be at risk for OSA, but he had not received a formal diagnosis. The patient met many of the criteria for screening OSA that are listed for a STOP-Bang Questionnaire.1 He was clinically and serologically euthyroid. Neck ultrasound revealed a very large thyroid mass with cystic and solid lesions throughout. Other than hoarseness, the patient reported no compressive symptoms, such as dysphagia or airway compromise. He was maintained on metoprolol, fosinopril, a thiazide for hypertension, and metformin and insulin for diabetes. A physical examination was remarkable for a Mallampati IV airway classification, a 61-cm neck circumference, 177 cm height, 142 kg weight, and a body mass index of 45. These preoperative assessments were predictive of a high probability of very difficult mask ventilation and intubation after the induction of a general anesthetic, or in any other situation requiring tracheal intubation, such as respiratory failure in the postoperative period.
Preoperative laboratory studies, chest radiograph, and electrocardiogram (ECG) were unremarkable. Computed tomography (CT) imaging of the neck revealed marked enlargement of the thyroid, which had a multinodular, heterogeneous appearance with scattered calcifications. The left lobe of the thyroid measured 13.0 cm craniocaudal by 9.47 cm transverse by 6.8 cm anteroposterior. The right lobe of the thyroid measured 12.0 cm craniocaudal by 7.6 cm transverse by 7.0 cm anteroposterior (Figure 1).
The first concern for this patient was a secure airway, which potentially could have been very difficult to procure with a standard IV induction of anesthesia followed by a direct laryngoscopy. This was further constrained by the surgical requirement that the patient be intubated with an electromyography (EMG) endotracheal tube for monitoring of the recurrent laryngeal nerves, as thyroid surgery carries the risk of injury to these nerves. The type of tube that was used had a larger diameter than that of a standard endotracheal tube (the EMG tube measured 10.2 mm outside diameter vs 9.6 mm outside diameter for a standard tube) but was also far more rigid, precluding nasal intubation and making navigation of the tip around corners and obstructions more difficult. A final laryngoscopy was also needed for confirmation of optimal electrode placement at the vocal cord level (Figure 2).
The anesthetic plan was to secure the airway with an awake oral fiberoptic intubation under sedation and topical local anesthetic to avoid the hypoxemia that would ensue if the patient lost spontaneous respiration. The patient was brought without preoperative sedation to the operating room, standard monitors (eg, ECG, noninvasive blood pressure, pulse oximetry) were applied and IV access was obtained. Blood pressure, heart rate, and oxygen saturation were within normal limits. He was placed on oxygen 2 L/min by nasal cannula and given a 1 μg/kg loading dose of dexmedetomidine over 10 minutes and thereafter maintained on a 0.4 μg/kg/h maintenance infusion during the entire airway intubation sequence. A topical anesthesia of 4% lidocaine spray was applied to the upper airway, and a transtracheal injection was performed with 2 mL of 4% lidocaine. The patient’s anatomy precluded the use of superior laryngeal nerve blocks. During the dexmedetomidine loading, he was given 1 mg midazolam and 100 μg fentanyl IV incrementally. No significant hemodynamic or respiratory changes occurred with this sedation regimen.
An attempt to place an oral intubation bite block failed, because the stiff EMG tube proved too difficult to pass through it. Therefore, the EMG tube and rolled gauze pads placed between the upper and lower teeth were used to protect the fiberoptic bronchoscope while it was guided past the base of the tongue. As was noted in the CT scan, the airway was deviated slightly to the left, and this information was useful for guiding the fiberscope. The location of the epiglottis was fairly difficult to ascertain due to redundant tissue in the hypopharyngeal area but was ultimately visible through the fiberscope.
The vocal cords were not visible, possibly due to the significant amount of airway edema and/or redundant tissue between the epiglottis and the vocal cords: Only the space beneath the epiglottis could be seen via the fiberscope. Passing the bronchoscope through the larynx also was problematic due to what may be described as altered spatial/angular relationships and due to the supraglottic edema/tissue leaving little room for the tip of the bronchoscope to be maneuvered. Figure 3 shows a CT scan image of the supraglottic area.
It took 45 minutes and multiple attempts to pass the bronchoscope into the trachea. The dexmedetomidine infusion was continued throughout this entire sequence. The patient tolerated this manipulation with little difficulty, despite the multiple airway maneuvers, and his hemodynamic and respiratory status remained clinically stable. Oxygen saturation was 95% to 100% during this sequence and the patient did not show evidence of significant upper airway collapse, desaturation, or apnea, which are sometimes encountered during sedation for airway manipulation.
The patient’s hemodynamic status remained near baseline values throughout the airway manipulation. The patient never lost his ability to cooperate. After manipulation of the fiberscope into the trachea, the tracheal rings and carina were visualized, and the tube was advanced over the scope. Minimal to mild coughing occurred once the tube passed through the vocal cords. The tube position in the trachea was verified with end-tidal CO2 and bronchoscopy and then the induction of anesthesia with propofol was completed. A laryngoscopy using a videolaryngoscope confirmed proper EMG electrode placement. Large-bore IV access and an arterial line were then secured.
The operation lasted about 15 hours. Maintenance of anesthesia was accomplished with the use of the volatile anesthetic desflurane, titrated to patient response to the surgical procedure. Additionally, 550 μg of IV fentanyl was used intermittently during the operation. Dexmedetomidine was infused at a rate of 0.2 to 0.4 μg/kg/h during the anesthetic, titrated to hemodynamic response. All hemodynamic parameters remained stable and within 20% of preoperative levels during the procedure. The blood loss during the procedure was minimal (< 100 mL), and acceptable readings from the EMG tube were confirmed throughout the surgical procedure.
The 686-gram thyroid mass was confirmed to be a multinodular goiter. Due to the difficulty with intubation, the length of the surgical procedure, and the likelihood of airway difficulties from edema possibly requiring reintubation, the patient was left intubated and mechanically ventilated overnight and sedated with a dexmedetomidine infusion of 0.3 μg/kg/h and propofol 35 mL/h. No further medications were required. He tolerated the ventilator without fighting, straining, coughing, or hypertensive responses and remained cooperative when aroused. He was successfully extubated the following day. Afterward, the patient maintained his airway and had only a mild right vocal cord paresis complicating his surgical management.
Discussion
The critical issues associated with this successful endotracheal intubation included the patient’s obesity, thyroid mass size, and deviation/compression of the trachea. Were this patient morbidly obese only, airway management would still be problematic; this was exacerbated by the concurrent pathologies. Dexmedetomidine possesses several advantageous properties for the perioperative period and was chosen as sedation for the awake intubation due to its sedative-analgesic effects, opioid sparing effects, lack of respiratory depression, maintenance of patient cooperation, and antisialagogue effect.2-5 Dexmedetomidine has previously been shown to be useful for awake intubation of difficult airway cases.4,6,7 Importantly, the dexmedetomidine sedation seemed to blunt the hypertensive responses often seen during airway maneuvers.8 It was also chosen as an intraoperative adjunct due to the above-noted opioid-sparing effects, given the importance of minimizing perioperative opioids needed for this morbidly obese individual with airway compromise.
In the literature, dexmedetomidine has been shown to greatly reduce the need for opioids, both intraoperatively and immediately postoperatively in many citations, for example, in postoperative mechanically ventilated coronary artery bypass graft patients.2,9 Opioid usage reduction is especially needed for the morbidly obese who are at increased risk of OSA and the attendant increased sensitivity to the respiratory depressant effects of narcotics. Postoperative opioids are being debated in the literature as potentially being a risk factor for cancer recurrence due to the effects on the immune system.5,10
Although the pathology report was benign for this patient, it was thought that prior to the surgery a reduction in opioid usage was important because he may have had a thyroid carcinoma in addition to the other respiratory considerations. Additionally, it was desired to decrease the amount of volatile anesthetic agents needed for this patient, because the surgical procedure was anticipated to be quite prolonged (it lasted 15 hours).
Conclusions
Recent research showed that a dexmedetomidine infusion combined with a low-dosage midazolam was superior to a higher dosage midazolam regimen for awake fiberoptic intubation in terms of stability, comfort, cooperation, and patient satisfaction.11 This is an example of the utility of dexmedetomidine. It is often insufficient when used alone, but as an adjunct will markedly reduce the dosage of other sedatives needed to achieve the desired Ramsey sedation scores and/or clinical benefit. Additionally, dexmedetomidine has been shown to facilitate weaning patients in a case series (who had previously failed weaning) from mechanical ventilation in surgical intensive care settings.12
The use of dexmedetomidine facilitated awake intubation for this patient and was helpful for postoperative sedation. The authors believe that dexmedetomidine has potential benefits in all phases of surgery and is a potentially valuable addition to the anesthesiologist’s and intensivist’s armamentarium.
Acknowledgements
This work was conducted at and supported by the G.V. (Sonny) Montgomery VA Medical Center in Jackson, Mississippi.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The following case report describes the use of dexmedetomidine as the primary sedative for an awake endotracheal intubation, as an adjuvant for general anesthesia, and for postoperative sedation for mechanical ventilation. This case illustrates problems that attracted the attention of federal institutions, specifically the management of difficult airways (with and without anatomic distortion), obesity, and obstructive sleep apnea (OSA). As such, it is of potential interest not only to anesthesiologists, but also other health care providers in the VA, especially those who might practice in intensive care settings.
Dexmedetomidine has useful pharmacologic properties that have potential use in a wide variety of clinical scenarios. Dexmedetomidine is currently indicated for sedation in nonintubated patients before and during surgical and other procedures and in intubated and mechanically ventilated patients during treatment in an intensive care setting.
Large neck masses can produce numerous problems that complicate the anesthetic management in the intraoperative and immediate postoperative arenas. The adjuvant use of dexmedetomidine, an alpha-2 agonist that has useful properties for both the anesthetic and intensive care situations, will be discussed. The problems involved with the management and resection of large neck masses include tracheal deviation, tracheal compression, airway edema, distorted anatomy, difficult mask ventilation, difficult intubation, postoperative recurrent laryngeal nerve dysfunction, and difficult exposure for tracheostomy.
Case Report
A 46-year-old man was referred for removal of a large thyroid mass. His past medical history included hypertension, obesity, and type 2 diabetes mellitus. Clinically, the patient seemed to be at risk for OSA, but he had not received a formal diagnosis. The patient met many of the criteria for screening OSA that are listed for a STOP-Bang Questionnaire.1 He was clinically and serologically euthyroid. Neck ultrasound revealed a very large thyroid mass with cystic and solid lesions throughout. Other than hoarseness, the patient reported no compressive symptoms, such as dysphagia or airway compromise. He was maintained on metoprolol, fosinopril, a thiazide for hypertension, and metformin and insulin for diabetes. A physical examination was remarkable for a Mallampati IV airway classification, a 61-cm neck circumference, 177 cm height, 142 kg weight, and a body mass index of 45. These preoperative assessments were predictive of a high probability of very difficult mask ventilation and intubation after the induction of a general anesthetic, or in any other situation requiring tracheal intubation, such as respiratory failure in the postoperative period.
Preoperative laboratory studies, chest radiograph, and electrocardiogram (ECG) were unremarkable. Computed tomography (CT) imaging of the neck revealed marked enlargement of the thyroid, which had a multinodular, heterogeneous appearance with scattered calcifications. The left lobe of the thyroid measured 13.0 cm craniocaudal by 9.47 cm transverse by 6.8 cm anteroposterior. The right lobe of the thyroid measured 12.0 cm craniocaudal by 7.6 cm transverse by 7.0 cm anteroposterior (Figure 1).
The first concern for this patient was a secure airway, which potentially could have been very difficult to procure with a standard IV induction of anesthesia followed by a direct laryngoscopy. This was further constrained by the surgical requirement that the patient be intubated with an electromyography (EMG) endotracheal tube for monitoring of the recurrent laryngeal nerves, as thyroid surgery carries the risk of injury to these nerves. The type of tube that was used had a larger diameter than that of a standard endotracheal tube (the EMG tube measured 10.2 mm outside diameter vs 9.6 mm outside diameter for a standard tube) but was also far more rigid, precluding nasal intubation and making navigation of the tip around corners and obstructions more difficult. A final laryngoscopy was also needed for confirmation of optimal electrode placement at the vocal cord level (Figure 2).
The anesthetic plan was to secure the airway with an awake oral fiberoptic intubation under sedation and topical local anesthetic to avoid the hypoxemia that would ensue if the patient lost spontaneous respiration. The patient was brought without preoperative sedation to the operating room, standard monitors (eg, ECG, noninvasive blood pressure, pulse oximetry) were applied and IV access was obtained. Blood pressure, heart rate, and oxygen saturation were within normal limits. He was placed on oxygen 2 L/min by nasal cannula and given a 1 μg/kg loading dose of dexmedetomidine over 10 minutes and thereafter maintained on a 0.4 μg/kg/h maintenance infusion during the entire airway intubation sequence. A topical anesthesia of 4% lidocaine spray was applied to the upper airway, and a transtracheal injection was performed with 2 mL of 4% lidocaine. The patient’s anatomy precluded the use of superior laryngeal nerve blocks. During the dexmedetomidine loading, he was given 1 mg midazolam and 100 μg fentanyl IV incrementally. No significant hemodynamic or respiratory changes occurred with this sedation regimen.
An attempt to place an oral intubation bite block failed, because the stiff EMG tube proved too difficult to pass through it. Therefore, the EMG tube and rolled gauze pads placed between the upper and lower teeth were used to protect the fiberoptic bronchoscope while it was guided past the base of the tongue. As was noted in the CT scan, the airway was deviated slightly to the left, and this information was useful for guiding the fiberscope. The location of the epiglottis was fairly difficult to ascertain due to redundant tissue in the hypopharyngeal area but was ultimately visible through the fiberscope.
The vocal cords were not visible, possibly due to the significant amount of airway edema and/or redundant tissue between the epiglottis and the vocal cords: Only the space beneath the epiglottis could be seen via the fiberscope. Passing the bronchoscope through the larynx also was problematic due to what may be described as altered spatial/angular relationships and due to the supraglottic edema/tissue leaving little room for the tip of the bronchoscope to be maneuvered. Figure 3 shows a CT scan image of the supraglottic area.
It took 45 minutes and multiple attempts to pass the bronchoscope into the trachea. The dexmedetomidine infusion was continued throughout this entire sequence. The patient tolerated this manipulation with little difficulty, despite the multiple airway maneuvers, and his hemodynamic and respiratory status remained clinically stable. Oxygen saturation was 95% to 100% during this sequence and the patient did not show evidence of significant upper airway collapse, desaturation, or apnea, which are sometimes encountered during sedation for airway manipulation.
The patient’s hemodynamic status remained near baseline values throughout the airway manipulation. The patient never lost his ability to cooperate. After manipulation of the fiberscope into the trachea, the tracheal rings and carina were visualized, and the tube was advanced over the scope. Minimal to mild coughing occurred once the tube passed through the vocal cords. The tube position in the trachea was verified with end-tidal CO2 and bronchoscopy and then the induction of anesthesia with propofol was completed. A laryngoscopy using a videolaryngoscope confirmed proper EMG electrode placement. Large-bore IV access and an arterial line were then secured.
The operation lasted about 15 hours. Maintenance of anesthesia was accomplished with the use of the volatile anesthetic desflurane, titrated to patient response to the surgical procedure. Additionally, 550 μg of IV fentanyl was used intermittently during the operation. Dexmedetomidine was infused at a rate of 0.2 to 0.4 μg/kg/h during the anesthetic, titrated to hemodynamic response. All hemodynamic parameters remained stable and within 20% of preoperative levels during the procedure. The blood loss during the procedure was minimal (< 100 mL), and acceptable readings from the EMG tube were confirmed throughout the surgical procedure.
The 686-gram thyroid mass was confirmed to be a multinodular goiter. Due to the difficulty with intubation, the length of the surgical procedure, and the likelihood of airway difficulties from edema possibly requiring reintubation, the patient was left intubated and mechanically ventilated overnight and sedated with a dexmedetomidine infusion of 0.3 μg/kg/h and propofol 35 mL/h. No further medications were required. He tolerated the ventilator without fighting, straining, coughing, or hypertensive responses and remained cooperative when aroused. He was successfully extubated the following day. Afterward, the patient maintained his airway and had only a mild right vocal cord paresis complicating his surgical management.
Discussion
The critical issues associated with this successful endotracheal intubation included the patient’s obesity, thyroid mass size, and deviation/compression of the trachea. Were this patient morbidly obese only, airway management would still be problematic; this was exacerbated by the concurrent pathologies. Dexmedetomidine possesses several advantageous properties for the perioperative period and was chosen as sedation for the awake intubation due to its sedative-analgesic effects, opioid sparing effects, lack of respiratory depression, maintenance of patient cooperation, and antisialagogue effect.2-5 Dexmedetomidine has previously been shown to be useful for awake intubation of difficult airway cases.4,6,7 Importantly, the dexmedetomidine sedation seemed to blunt the hypertensive responses often seen during airway maneuvers.8 It was also chosen as an intraoperative adjunct due to the above-noted opioid-sparing effects, given the importance of minimizing perioperative opioids needed for this morbidly obese individual with airway compromise.
In the literature, dexmedetomidine has been shown to greatly reduce the need for opioids, both intraoperatively and immediately postoperatively in many citations, for example, in postoperative mechanically ventilated coronary artery bypass graft patients.2,9 Opioid usage reduction is especially needed for the morbidly obese who are at increased risk of OSA and the attendant increased sensitivity to the respiratory depressant effects of narcotics. Postoperative opioids are being debated in the literature as potentially being a risk factor for cancer recurrence due to the effects on the immune system.5,10
Although the pathology report was benign for this patient, it was thought that prior to the surgery a reduction in opioid usage was important because he may have had a thyroid carcinoma in addition to the other respiratory considerations. Additionally, it was desired to decrease the amount of volatile anesthetic agents needed for this patient, because the surgical procedure was anticipated to be quite prolonged (it lasted 15 hours).
Conclusions
Recent research showed that a dexmedetomidine infusion combined with a low-dosage midazolam was superior to a higher dosage midazolam regimen for awake fiberoptic intubation in terms of stability, comfort, cooperation, and patient satisfaction.11 This is an example of the utility of dexmedetomidine. It is often insufficient when used alone, but as an adjunct will markedly reduce the dosage of other sedatives needed to achieve the desired Ramsey sedation scores and/or clinical benefit. Additionally, dexmedetomidine has been shown to facilitate weaning patients in a case series (who had previously failed weaning) from mechanical ventilation in surgical intensive care settings.12
The use of dexmedetomidine facilitated awake intubation for this patient and was helpful for postoperative sedation. The authors believe that dexmedetomidine has potential benefits in all phases of surgery and is a potentially valuable addition to the anesthesiologist’s and intensivist’s armamentarium.
Acknowledgements
This work was conducted at and supported by the G.V. (Sonny) Montgomery VA Medical Center in Jackson, Mississippi.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Hathaway B, Johnson JT. Safety of uvulopalatopharyngoplasty as outpatient surgery. Otolaryngol Head Neck Surg. 2006;134(4):542-544.
2. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cen). 2001;14(1):13-21.
3. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53(7):646-652.
4. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382-394.
5. Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136-1142.
6. Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19(4):141-144.
7. Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: A report of three cases. J Clin Anesth. 2004;16(2):124-126.
8. Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7(1):43-52.
9. Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576-584.
10. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109(2):180-187.
11. Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22(1):35-40.
12. Siobal MS, Kallet RH, Kivett VA, Tang JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir Care. 2006;51(5):492-496.
1. Hathaway B, Johnson JT. Safety of uvulopalatopharyngoplasty as outpatient surgery. Otolaryngol Head Neck Surg. 2006;134(4):542-544.
2. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cen). 2001;14(1):13-21.
3. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53(7):646-652.
4. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382-394.
5. Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136-1142.
6. Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19(4):141-144.
7. Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: A report of three cases. J Clin Anesth. 2004;16(2):124-126.
8. Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: Perioperative haemodynamics and anaesthetic requirements. Drugs R D. 2006;7(1):43-52.
9. Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576-584.
10. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109(2):180-187.
11. Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22(1):35-40.
12. Siobal MS, Kallet RH, Kivett VA, Tang JF. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir Care. 2006;51(5):492-496.