User login
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
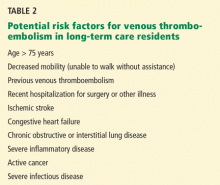
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
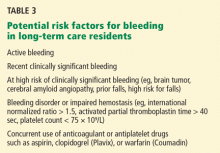
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
KEY POINTS
- Assessment of VTE risk and consideration of need for anticoagulant prophylaxis in long-term care residents are based on indirect data, derived primarily from studies of acutely ill hospitalized medical patients.
- Drugs and devices for thromboprophylaxis have been studied in medical and surgical populations, but issues of efficacy and safety are likely to also pertain to long-term care residents.
- Thromboprophylaxis should be considered for long-term care residents if they are definitely at increased risk of VTE—ie, if they have an acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease; acute inflammatory disease; acute infection; active cancer; or immobility and prior VTE.