User login
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
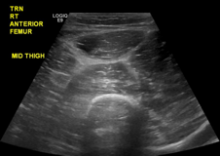
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
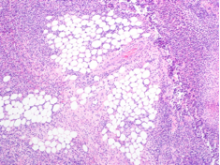
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
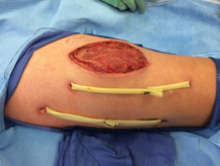
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.