User login
Introduction
The development of new cancer therapies relies on the successful development and completion of clinical trials. While clinical trials have led to significant improvements in cancer treatment, the success is dependent upon patient enrollment and participation. Unfortunately, fewer than 5% of adult patients enroll in trials.1-3 This represents a significant barrier to the development and approval of new cancer treatments. Reasons for low accrual into trials are multifactorial, but include structural barriers (eg, clinic access), clinical barriers (eg, eligibility criteria), and physician and patient attitudes towards trial enrollment.4,5 One study at the University of California Davis Cancer Center reported 49% of patients declined participation despite meeting eligibility criteria,3,6 suggesting that psychosocial barriers such as knowledge of trials and attitudes towards clinical research are a major impediment to accrual.7-9
Bone and soft tissue sarcoma represent a heterogeneous group of tumors of mesenchymal origin that are an important cause of morbidity and mortality. Local disease is often treated with a multidisciplinary approach including surgery, radiation, and systemic therapy. Metastatic disease is predominantly treated palliatively with systemic therapy.10 Given its rarity and heterogeneity, trial accrual is of particular importance in sarcoma and often requires multiple sites to enroll adequate numbers of patients. While sarcoma represents <1% of adult malignancies overall, it constitutes ~15% of malignancies in the adolescent and young adult (AYA) population (15- 39 years old).11,12 Sarcoma represents a patient population in which low trial accrual has been correlated with lack of progress in cancer-related outcomes in both the adult and AYA populations.13 The reasons for low accrual rates among patients with sarcoma are poorly understood.
Sarcomas represent a molecularly and biologically heterogeneous group of malignancies with over 100 different subtypes.12 As a result, there has been significant interest in performing molecular profiling, or genetic sequencing, to identify “targetable” mutations. Targetable mutations refer to a specific genetic change identified within the tumor molecular profile for which there is a specific drug that may demonstrate activity against a particular tumor. Given the widespread utilization of this technology in sarcoma, identifying and understanding patient perceptions with regard to molecular profiling is critically important in this disease.14
In this study, we use a cross-sectional design to describe patient perceptions of trial enrollment among patients with bone and soft tissue sarcoma through validated measures, including attitudes towards clinical trials, knowledge of clinical trials, and perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about clinical trial participation, receptivity to learning more about clinical trials, and willingness to participate in clinical trials.6 In addition, we describe this patient cohort’s perceptions of molecular profiling, as current and future trials are increasingly driven by molecular or other biomarkers.
Methods
This was a cross-sectional electronic survey study of patients with bone and soft tissue sarcoma treated at Northwestern Medicine (NM) over a 5-year period. NM Enterprise Data Warehouse (NMEDW) is a single, comprehensive, and integrated repository of all clinical and research data sources within NM. The study was approved by the Northwestern University Institutional Review Board.
Survey
The investigators designed a self-administered, online survey, which was built using Research Electronic Data Capture (REDCap). The survey consisted of three sections that were answered using skip logic—a custom path through the survey that varied based on patients’ answers: (1) Patient demographic information and trial perceptions (answered by all patients); (2) Thoughts about molecular profiling (answered by patients who answered “yes” to the question, “Have you heard about molecular profiling of tumors?”); and (3) Considerations to undergo molecular profiling (answered by patients who answered “yes” to the question, “Have you undergone profiling of your cancer?”).
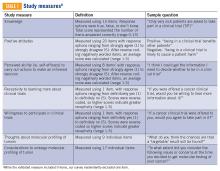
Clinical trial perceptions included questions assessing (1) patient knowledge about trials; (2) patient attitudes toward trials; (3) perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about trial participation; (4) receptivity to learning more about trials; and (5) willingness to participate in trials. These outcome measures had been previously developed and pilot tested for reliability and validity (TABLE 1).6
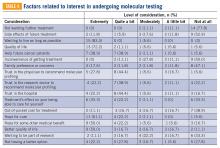
Thoughts about molecular profiling of tumors were assessed using nine items (TABLE 1). Of these, items assessing potential benefit or harm of molecular profiling were assessed using a 7-step Likert scale. Items assessing maximal benefit or harm of therapy, importance of quality vs length of life, and concern about the cost of molecular testing were assessed using a 5-step Likert scale. The study team developed and piloted these questions because there is no validated survey assessing these domains.
Considerations to undergo molecular profiling were assessed using 17 items. Items were in response to the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” Responses were assessed using a 5-point Likert scale.
Data Collection
Patients 18 years and older evaluated at NM between November 20, 2012, and November 20, 2017, with a diagnosis of sarcoma were identified by query of the NMEDW by ICD-10 codes (C40, C41.9, C44.99, C45-49, C55, C71.9, D48, D49.9, and M12.20) or equivalent ICD-9 codes. Patients were subsequently excluded if they did not have a diagnosis of bone or soft tissue sarcoma, no e-mail address listed, had died, or had not been evaluated at an NM clinic in the previous 5 years. Patients with a diagnosis of gastrointestinal stromal tumor and Kaposi’s sarcoma were also excluded.
A personalized contact e-mail was sent to patients containing an explanation of the survey and an internet link to the electronic survey through REDCap from January 2018 to March 2018. If patients did not respond to the survey, two follow-up reminder e-mails were sent 2 and 4 days following the initial survey. The link was protected so that each patient could complete the survey only once. Responses were collected through the REDCap platform. Patients read and signed an electronic consent form prior to completing the survey.
Upon completion of the survey, patients were offered a $50 VISA gift card as compensation, with an option to donate their compensation to the Robert H. Lurie Comprehensive Cancer Center Sarcoma Research Fund.
Over the described survey period, open clinical trials for patients with bone and soft tissue sarcoma available at NM were evaluated. The number of patients screened and accrued to each trial were recorded.
Statistical analysis
Responses were separated from the personal data for complete anonymization. Descriptive statistical analysis was performed for demographics and disease variables and were summarized using frequencies and percentages. Median and range were used for age. Correlations between continuous variables were analyzed using Spearman correlations. Scores were compared between subgroups using the Mann-Whitney test. Descriptive statistics for knowledge, attitude, and ability scores include means and 95% confidence intervals. Correlations were interpreted as small (r=0.10), medium (r=0.30), or large (r=0.50).15 Statistical significance was indicated when P<0.05.
Results
Patients
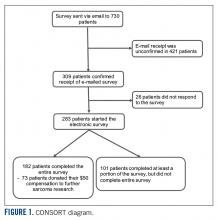
Seven hundred fifty patients were eligible to participate in the survey and received the initial and two follow-up e-mails. Twenty e-mailed surveys bounced back. Three hundred nine patients opened the initial e-mail and 283 patients (37.7% of total and 91.6% of opened) completed at least a portion of the survey, with 182 patients completing the entire survey (FIGURE 1). Data for analysis were used from patients who completed at least a portion of the survey.
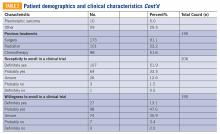
Baseline characteristics of patients who responded can be seen in TABLE 2. Patients had a median age of 56, the majority were female (59.4%), white (88.2%), and most had college or university graduate degrees or higher educational level (69.0%). Patients had various different histological subtypes, with the most common being liposarcoma (16.5%) and leiomyosarcoma (16.0%). Slightly more than a quarter (26.8%) of patients had metastatic disease, and 84.2% had never been enrolled in a clinical trial. Previous treatments included surgery (91.1%), radiation (53.2%), and chemotherapy (51.6%). Prior to completing the survey, 85.4% reported being receptive to a cancer clinical trial, while 60.7% of patients reported willingness to participate in a clinical trial.
Knowledge, attitudes, and perceived ability
A statistically significant correlation was observed between greater knowledge of trials and more positive attitudes towards trials (P<0.001; r=0.5, FIGURE 2A). In relating patient attitudes with perceived ability, again a significant correlation was seen (P<0.001; r=0.4, FIGURE 2B). In contrast, knowledge had a weak correlation with perceived ability (P=0.024; r=0.2, FIGURE 2C). There was no difference regarding patient knowledge, attitudes, or perceived ability by age, gender, race, or income.
Knowledge, attitudes, perceived ability, and clinical trial enrollment
Thirty patients reported clinical trial experience (either previously or currently enrolled in trials) and 160 patients were never enrolled. Of the 30 patients with trial experience, 7 reported being currently enrolled, while 23 reported previous enrollment. Of these patients, 16 had metastatic disease, while 12 had non-metastatic disease, and 2 were unsure whether or not they had metastatic disease.
Patients with previous clinical trial exposure (currently or previously enrolled in clinical trials) demonstrated significantly greater trial knowledge, with a mean knowledge score of 9.3 (CI 8.5-10.0) compared with 7.7 (CI 7.3-8.1) among patients without trial exposure (P=0.002; FIGURE 3A). Similarly, patients with trial experience also had statistically significant more positive attitudes towards trials as compared with patients with no trial experience, with a mean attitude score of 3.8 (CI 3.6-4.0) and 3.5 (CI 3.4-3.6), respectively (P=0.001; FIGURE 3B). While numerically patients with trial experience have greater perceived ability compared with patients with no trial experience, with a mean score of 4.4 (CI 4.2-4.6) and 4.2 (CI 4.1-4.3), respectively, this difference did not reach statistical significance (FIGURE 3C).
Knowledge, attitudes, perceived ability, and disease stage
An analysis was performed comparing patients with metastatic vs non-metastatic disease. It was observed that patients with metastatic disease had similar knowledge of trials compared with non-metastatic patients, with a mean knowledge score of 8.4 (CI 7.7- 9.1) and 7.9 (CI 7.5-8.4), respectively, (P=0.3; FIGURE 4A). In contrast, patients with metastatic disease had more positive attitudes compared with non-metastatic patients, with a mean score of 3.7 (CI 3.5-3.8) and 3.5 (CI 3.4-3.6), respectively, which was statistically significant (P=0.03; FIGURE 4B). There was no difference in perceived ability in metastatic vs non-metastatic patients (FIGURE 4C).
Thoughts about molecular profiling
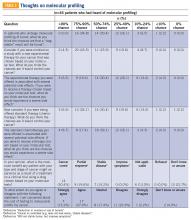
Of the total number of patients, 46 patients had heard of molecular profiling and were presented with questions regarding their thoughts (TABLE 3). Approximately two-thirds (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their tumor molecular profile. A majority (71.7%) of patients thought that a new experimental therapy chosen based on a patient’s tumor molecular profile would have at least a 50% chance of controlling the cancer. Somewhat less than a third (30.4%) of patients thought that total cure is the maximal benefit a patient could experience as a result of a treatment on a clinical trial using a drug chosen based on molecular tests. About half (52.2%) of patients agreed with the statement, “I am concerned about the cost of the test to molecularly profile my cancer.”
Considerations to undergo molecular profiling
Eighteen patients had undergone molecular profiling of their tumor. These patients were posed the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” (TABLE 4). A majority (83.3%) of patients stated that wanting to live as long as possible was important, and 72.2% of patients stated that quality of life was important. A majority (83.3%) of patients stated that hope for a cure was an extremely or quite a bit important consideration. Helping future cancer patients was extremely or quite a bit important for 77.8% of patients, while wanting to be a part of research was not at all or of little importance in 50.0% of patients.
Clinical trials at NM
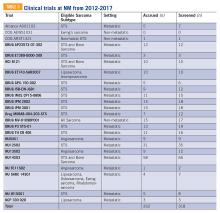
Twenty-four clinical trials were available at NM between the years 2012 and 2017 for patients with bone and soft tissue sarcoma. Of these trials, 3 of 24 were for non-metastatic patients, while the remaining 21 were open only to metastatic patients. The median number of patients screened per trial was 11 (range 0-66) and the median number of patients accrued per trial was 9 (range 0-58). Of the 24 trials, 17 were not subtype specific (13 included soft tissue sarcoma alone while 4 included both bone and soft tissue sarcoma). The remaining 7 trials were sarcoma subtype specific (eg, angiosarcoma, liposarcoma, etc). Trials available at NM during this period are included in TABLE 5. There were 318 patients screened and 262 patients accrued to sarcoma trials over this time period, with a screen failure rate of 17.6% overall.
Discussion
Our study sought to describe perceptions of clinical trial enrollment among patients with bone and soft tissue sarcoma in order to elucidate and overcome barriers to enrollment, which to the best of our knowledge had not been previously described. Using previously validated patient- reported outcomes in the literature,6 our data reveal a correlation between knowledge of trials and more positive attitudes towards trials. This underscores the importance of awareness and educational strategies in this cancer population as a whole. Interventions should focus on patient perceptions that contribute to lack of participation, such as fear of side effects, loss of control (eg, idea of placebo or randomization), logistical challenges (eg, additional time or convenient location), and cost.3,5,7,16,17 For example, patients concerned about randomization should be educated on equipoise and other ethical considerations in trial design.5 Previous research has suggested that a multimedia psychoeducational intervention was effective in improving attitudes toward trials.6 Educating patients on the essential role of trials in oncology care, as demonstrated by the vast number of new drug approvals in recent years, is an essential strategy to improve attitudes, and subsequently leads to higher patient accrual rates.
In our study, both knowledge and at titudes were increased in patients with previous trial exposure. This suggests that either patients with greater knowledge and more positive attitudes are more likely to enroll in trials, or that patients with direct trial exposure are more knowledgeable and develop more positive attitudes. Our patient population was overall receptive to learning more about clinical trials (85.4%) and willing to participate (65.8%). At the same time, our study demonstrated low to medium correlations between attitudes and perceived ability to take steps towards making an informed decision to enroll in a trial (r=0.4), which may partially be explained by the absence of a tangible trial opportunity. While we did not assess specifically whether patients in our study were offered a trial, there was a substantial trial menu at NM with limited screen failures and decent trial accrual over the time frame of our study. This underscores the importance not only of patient-focused strategies to increase educational and attitudinal resources, but also a need to focus on research-site optimization that includes opening of multiple trials in various settings, systematic pre-screening of patients, and eligibility criteria that are inclusive and rational.5
The patient population with metastatic disease demonstrated more positive attitudes towards enrolling in clinical trials. This cohort accrued well to clinical trials, with 21 of 24 trials enrolling specifically patients with metastatic disease. Of the patients who responded to our survey, patients with previous trial exposure were enriched for patients with metastatic disease (53.3% metastatic among previous trial exposure versus 26.8% metastatic overall). These observations are likely reflective of the need for novel therapies in this disease setting. At the same time, approximately 25% of patients with localized soft tissue sarcoma will develop distant metastatic disease after successful treatment of their primary tumor, which increases to 40% to 50% in larger and higher-grade tumors.18 Three of 24 trials were open for patients with non-metastatic disease, of which one managed to accrue patients. Patients with non-metastatic disease had more negative attitudes towards trial enrollment. These disproportionate findings suggest a need for interventions to increase patient awareness and attitudes towards trial enrollment among this patient population and the importance of research-site optimization for trial opportunities across disease states.
Molecular profiling of tumors and biomarker identification has become a critical component of further characterizing cancer subtypes. In our study, a majority (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their molecular profile. Molecular data on 5,749 bone and soft tissue sarcomas suggested that 9.5% of tumors demonstrate a “targetable result,” defined as a new molecular finding for which there is an FDA-approved drug for malignancies other than sarcoma (eg, BRAF V600E, Her2, etc.),19 suggesting an overestimation in our patient cohort of the likelihood of benefit of molecular profiling. These results highlight that the growing use of molecular profiling has increased the need for educational and supportive resources to help patients understand the utility of molecular profiling and aid in shared decision-making surrounding the results.
At the same time, the importance of identifying a targetable mutation in patients with sarcoma cannot be understated. As a recent example of this paradigm, tumors that harbor fusions with the neurotrophic receptor tyrosine kinase 1, 2, or 3 (NTRK1, 2, and 3) have a high response rate (~75%) to drugs that target these fusions, such as larotrectinib and entrectinib.13 Molecular profiling and identification of predictive biomarkers in small patient subsets has led to great challenges in trial design and research-site optimization. Novel designs that incorporate molecular profiling,20 such as the Lung- MAP trial21 and NCI’s Molecular Analysis for Therapy Choice (MATCH) trial,22 are emerging to identify new therapies for small patient subsets. As a rare and increasingly heterogeneous cancer, sarcoma represents a paradigm to provide insight into optimizing patient perceptions and research enterprises to maximize clinical trial enrollment.
Some limitations of our study include a homogeneous and selected patient population that was predominantly Caucasian and highly educated. Therefore, these findings should not be extrapolated to other populations with barriers to trial accrual, such as lower socioeconomic or minority populations. The low response rate and failure of some to complete the survey may have introduced some bias. Additionally, our data include self-reported outcomes, which could have affected our results. Finally, the limited number of patients who had undergone or heard of molecular profiling limited our ability to draw definitive conclusions, and should be assessed in larger patient cohorts.
While our paper addresses a unique population—the sarcoma patient—similar themes and issues pertain to all oncology patients. A recent review was published in the American Society of Clinical Oncology Educational Book23 looking at methods to overcome barriers to clinical trial enrollment. Their paper clearly illustrates mechanisms to assist with overcoming financial burdens associated with cancer clinical trials, overcoming barriers as they relate to patient and clinician difficulty in coping with the uncertainty inherent in clinical trial participation, and highlight the role of a patient navigator in clinical trial participation.
Conclusions
Interventions aimed at increasing awareness, knowledge, and attitudes towards clinical trials among sarcoma patients may lead to increased trial enrollment and greater progress in cancer treatment in this population. In addition to patient- focused interventions, thoughtful and strategic clinical trial designs that allow for the development of biomarker- driven therapeutics, while at the same time optimizing patient accrual rates, should be developed. Evaluation of barriers to clinical trial enrollment and molecular profiling of tumors among bone and soft tissue sarcoma patients at an academic center can serve as a paradigm to overcome barriers to enrollment in the era of an increasingly heterogeneous cancer population. TSJ
1. Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106(2):426-433.
2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004; 291(22):2720-2726.
3. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728-1733.
4. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185-198.
5. Cancer Action Network American Cancer Society. Barriers to patient enrollment in therapeutic clinical trials for cancer. 2018: https:// www.fightcancer.org/policy-resources/clinical- trial-barriers#figures. Accessed March 14, 2019.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw. 2007;5(8):655-664.
8. Cox K, McGarry J. Why patients don’t take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care (Engl). 2003;12(2): 114-122.
9. Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta- analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141-148.
10. National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 4.2019). https:// www.nccn.org/professionals/physician_gls/ pdf/sarcoma.pdf. Accessed October 30, 2019.
11. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
12. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncol. 2018(2):1-4.
13. Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891-1897.
14. Gornick MC, Cobain E, Le LQ, et al. Oncologists’ use of genomic sequencing data to inform clinical management. JCO Precision Oncol. 2018(2):1-13.
15. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988.
16. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536- 542.
17. Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17(9):1180-1190.
18. Singhi EK, Moore DC, Muslimani A. Metastatic soft tissue sarcomas: a review of treatment and new pharmacotherapies. P T. 2018;43(7): 410-429.
19. Gounder MM, Ali SM, Robinson V, et al. Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J Clin Oncol. 2017;35(15_suppl):abstr 11001.
20. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62-70.
21. Steuer CE, Papadimitrakopoulou V, Herbst RS, et al. Innovative clinical trials: the LUNGMAP study. Clin Pharmacol Ther. 2015;97(5): 488-491.
22. McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. 2015;107(7).
23. Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105-114.
Introduction
The development of new cancer therapies relies on the successful development and completion of clinical trials. While clinical trials have led to significant improvements in cancer treatment, the success is dependent upon patient enrollment and participation. Unfortunately, fewer than 5% of adult patients enroll in trials.1-3 This represents a significant barrier to the development and approval of new cancer treatments. Reasons for low accrual into trials are multifactorial, but include structural barriers (eg, clinic access), clinical barriers (eg, eligibility criteria), and physician and patient attitudes towards trial enrollment.4,5 One study at the University of California Davis Cancer Center reported 49% of patients declined participation despite meeting eligibility criteria,3,6 suggesting that psychosocial barriers such as knowledge of trials and attitudes towards clinical research are a major impediment to accrual.7-9
Bone and soft tissue sarcoma represent a heterogeneous group of tumors of mesenchymal origin that are an important cause of morbidity and mortality. Local disease is often treated with a multidisciplinary approach including surgery, radiation, and systemic therapy. Metastatic disease is predominantly treated palliatively with systemic therapy.10 Given its rarity and heterogeneity, trial accrual is of particular importance in sarcoma and often requires multiple sites to enroll adequate numbers of patients. While sarcoma represents <1% of adult malignancies overall, it constitutes ~15% of malignancies in the adolescent and young adult (AYA) population (15- 39 years old).11,12 Sarcoma represents a patient population in which low trial accrual has been correlated with lack of progress in cancer-related outcomes in both the adult and AYA populations.13 The reasons for low accrual rates among patients with sarcoma are poorly understood.
Sarcomas represent a molecularly and biologically heterogeneous group of malignancies with over 100 different subtypes.12 As a result, there has been significant interest in performing molecular profiling, or genetic sequencing, to identify “targetable” mutations. Targetable mutations refer to a specific genetic change identified within the tumor molecular profile for which there is a specific drug that may demonstrate activity against a particular tumor. Given the widespread utilization of this technology in sarcoma, identifying and understanding patient perceptions with regard to molecular profiling is critically important in this disease.14
In this study, we use a cross-sectional design to describe patient perceptions of trial enrollment among patients with bone and soft tissue sarcoma through validated measures, including attitudes towards clinical trials, knowledge of clinical trials, and perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about clinical trial participation, receptivity to learning more about clinical trials, and willingness to participate in clinical trials.6 In addition, we describe this patient cohort’s perceptions of molecular profiling, as current and future trials are increasingly driven by molecular or other biomarkers.
Methods
This was a cross-sectional electronic survey study of patients with bone and soft tissue sarcoma treated at Northwestern Medicine (NM) over a 5-year period. NM Enterprise Data Warehouse (NMEDW) is a single, comprehensive, and integrated repository of all clinical and research data sources within NM. The study was approved by the Northwestern University Institutional Review Board.
Survey
The investigators designed a self-administered, online survey, which was built using Research Electronic Data Capture (REDCap). The survey consisted of three sections that were answered using skip logic—a custom path through the survey that varied based on patients’ answers: (1) Patient demographic information and trial perceptions (answered by all patients); (2) Thoughts about molecular profiling (answered by patients who answered “yes” to the question, “Have you heard about molecular profiling of tumors?”); and (3) Considerations to undergo molecular profiling (answered by patients who answered “yes” to the question, “Have you undergone profiling of your cancer?”).
Clinical trial perceptions included questions assessing (1) patient knowledge about trials; (2) patient attitudes toward trials; (3) perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about trial participation; (4) receptivity to learning more about trials; and (5) willingness to participate in trials. These outcome measures had been previously developed and pilot tested for reliability and validity (TABLE 1).6
Thoughts about molecular profiling of tumors were assessed using nine items (TABLE 1). Of these, items assessing potential benefit or harm of molecular profiling were assessed using a 7-step Likert scale. Items assessing maximal benefit or harm of therapy, importance of quality vs length of life, and concern about the cost of molecular testing were assessed using a 5-step Likert scale. The study team developed and piloted these questions because there is no validated survey assessing these domains.
Considerations to undergo molecular profiling were assessed using 17 items. Items were in response to the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” Responses were assessed using a 5-point Likert scale.
Data Collection
Patients 18 years and older evaluated at NM between November 20, 2012, and November 20, 2017, with a diagnosis of sarcoma were identified by query of the NMEDW by ICD-10 codes (C40, C41.9, C44.99, C45-49, C55, C71.9, D48, D49.9, and M12.20) or equivalent ICD-9 codes. Patients were subsequently excluded if they did not have a diagnosis of bone or soft tissue sarcoma, no e-mail address listed, had died, or had not been evaluated at an NM clinic in the previous 5 years. Patients with a diagnosis of gastrointestinal stromal tumor and Kaposi’s sarcoma were also excluded.
A personalized contact e-mail was sent to patients containing an explanation of the survey and an internet link to the electronic survey through REDCap from January 2018 to March 2018. If patients did not respond to the survey, two follow-up reminder e-mails were sent 2 and 4 days following the initial survey. The link was protected so that each patient could complete the survey only once. Responses were collected through the REDCap platform. Patients read and signed an electronic consent form prior to completing the survey.
Upon completion of the survey, patients were offered a $50 VISA gift card as compensation, with an option to donate their compensation to the Robert H. Lurie Comprehensive Cancer Center Sarcoma Research Fund.
Over the described survey period, open clinical trials for patients with bone and soft tissue sarcoma available at NM were evaluated. The number of patients screened and accrued to each trial were recorded.
Statistical analysis
Responses were separated from the personal data for complete anonymization. Descriptive statistical analysis was performed for demographics and disease variables and were summarized using frequencies and percentages. Median and range were used for age. Correlations between continuous variables were analyzed using Spearman correlations. Scores were compared between subgroups using the Mann-Whitney test. Descriptive statistics for knowledge, attitude, and ability scores include means and 95% confidence intervals. Correlations were interpreted as small (r=0.10), medium (r=0.30), or large (r=0.50).15 Statistical significance was indicated when P<0.05.
Results
Patients
Seven hundred fifty patients were eligible to participate in the survey and received the initial and two follow-up e-mails. Twenty e-mailed surveys bounced back. Three hundred nine patients opened the initial e-mail and 283 patients (37.7% of total and 91.6% of opened) completed at least a portion of the survey, with 182 patients completing the entire survey (FIGURE 1). Data for analysis were used from patients who completed at least a portion of the survey.
Baseline characteristics of patients who responded can be seen in TABLE 2. Patients had a median age of 56, the majority were female (59.4%), white (88.2%), and most had college or university graduate degrees or higher educational level (69.0%). Patients had various different histological subtypes, with the most common being liposarcoma (16.5%) and leiomyosarcoma (16.0%). Slightly more than a quarter (26.8%) of patients had metastatic disease, and 84.2% had never been enrolled in a clinical trial. Previous treatments included surgery (91.1%), radiation (53.2%), and chemotherapy (51.6%). Prior to completing the survey, 85.4% reported being receptive to a cancer clinical trial, while 60.7% of patients reported willingness to participate in a clinical trial.
Knowledge, attitudes, and perceived ability
A statistically significant correlation was observed between greater knowledge of trials and more positive attitudes towards trials (P<0.001; r=0.5, FIGURE 2A). In relating patient attitudes with perceived ability, again a significant correlation was seen (P<0.001; r=0.4, FIGURE 2B). In contrast, knowledge had a weak correlation with perceived ability (P=0.024; r=0.2, FIGURE 2C). There was no difference regarding patient knowledge, attitudes, or perceived ability by age, gender, race, or income.
Knowledge, attitudes, perceived ability, and clinical trial enrollment
Thirty patients reported clinical trial experience (either previously or currently enrolled in trials) and 160 patients were never enrolled. Of the 30 patients with trial experience, 7 reported being currently enrolled, while 23 reported previous enrollment. Of these patients, 16 had metastatic disease, while 12 had non-metastatic disease, and 2 were unsure whether or not they had metastatic disease.
Patients with previous clinical trial exposure (currently or previously enrolled in clinical trials) demonstrated significantly greater trial knowledge, with a mean knowledge score of 9.3 (CI 8.5-10.0) compared with 7.7 (CI 7.3-8.1) among patients without trial exposure (P=0.002; FIGURE 3A). Similarly, patients with trial experience also had statistically significant more positive attitudes towards trials as compared with patients with no trial experience, with a mean attitude score of 3.8 (CI 3.6-4.0) and 3.5 (CI 3.4-3.6), respectively (P=0.001; FIGURE 3B). While numerically patients with trial experience have greater perceived ability compared with patients with no trial experience, with a mean score of 4.4 (CI 4.2-4.6) and 4.2 (CI 4.1-4.3), respectively, this difference did not reach statistical significance (FIGURE 3C).
Knowledge, attitudes, perceived ability, and disease stage
An analysis was performed comparing patients with metastatic vs non-metastatic disease. It was observed that patients with metastatic disease had similar knowledge of trials compared with non-metastatic patients, with a mean knowledge score of 8.4 (CI 7.7- 9.1) and 7.9 (CI 7.5-8.4), respectively, (P=0.3; FIGURE 4A). In contrast, patients with metastatic disease had more positive attitudes compared with non-metastatic patients, with a mean score of 3.7 (CI 3.5-3.8) and 3.5 (CI 3.4-3.6), respectively, which was statistically significant (P=0.03; FIGURE 4B). There was no difference in perceived ability in metastatic vs non-metastatic patients (FIGURE 4C).
Thoughts about molecular profiling
Of the total number of patients, 46 patients had heard of molecular profiling and were presented with questions regarding their thoughts (TABLE 3). Approximately two-thirds (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their tumor molecular profile. A majority (71.7%) of patients thought that a new experimental therapy chosen based on a patient’s tumor molecular profile would have at least a 50% chance of controlling the cancer. Somewhat less than a third (30.4%) of patients thought that total cure is the maximal benefit a patient could experience as a result of a treatment on a clinical trial using a drug chosen based on molecular tests. About half (52.2%) of patients agreed with the statement, “I am concerned about the cost of the test to molecularly profile my cancer.”
Considerations to undergo molecular profiling
Eighteen patients had undergone molecular profiling of their tumor. These patients were posed the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” (TABLE 4). A majority (83.3%) of patients stated that wanting to live as long as possible was important, and 72.2% of patients stated that quality of life was important. A majority (83.3%) of patients stated that hope for a cure was an extremely or quite a bit important consideration. Helping future cancer patients was extremely or quite a bit important for 77.8% of patients, while wanting to be a part of research was not at all or of little importance in 50.0% of patients.
Clinical trials at NM
Twenty-four clinical trials were available at NM between the years 2012 and 2017 for patients with bone and soft tissue sarcoma. Of these trials, 3 of 24 were for non-metastatic patients, while the remaining 21 were open only to metastatic patients. The median number of patients screened per trial was 11 (range 0-66) and the median number of patients accrued per trial was 9 (range 0-58). Of the 24 trials, 17 were not subtype specific (13 included soft tissue sarcoma alone while 4 included both bone and soft tissue sarcoma). The remaining 7 trials were sarcoma subtype specific (eg, angiosarcoma, liposarcoma, etc). Trials available at NM during this period are included in TABLE 5. There were 318 patients screened and 262 patients accrued to sarcoma trials over this time period, with a screen failure rate of 17.6% overall.
Discussion
Our study sought to describe perceptions of clinical trial enrollment among patients with bone and soft tissue sarcoma in order to elucidate and overcome barriers to enrollment, which to the best of our knowledge had not been previously described. Using previously validated patient- reported outcomes in the literature,6 our data reveal a correlation between knowledge of trials and more positive attitudes towards trials. This underscores the importance of awareness and educational strategies in this cancer population as a whole. Interventions should focus on patient perceptions that contribute to lack of participation, such as fear of side effects, loss of control (eg, idea of placebo or randomization), logistical challenges (eg, additional time or convenient location), and cost.3,5,7,16,17 For example, patients concerned about randomization should be educated on equipoise and other ethical considerations in trial design.5 Previous research has suggested that a multimedia psychoeducational intervention was effective in improving attitudes toward trials.6 Educating patients on the essential role of trials in oncology care, as demonstrated by the vast number of new drug approvals in recent years, is an essential strategy to improve attitudes, and subsequently leads to higher patient accrual rates.
In our study, both knowledge and at titudes were increased in patients with previous trial exposure. This suggests that either patients with greater knowledge and more positive attitudes are more likely to enroll in trials, or that patients with direct trial exposure are more knowledgeable and develop more positive attitudes. Our patient population was overall receptive to learning more about clinical trials (85.4%) and willing to participate (65.8%). At the same time, our study demonstrated low to medium correlations between attitudes and perceived ability to take steps towards making an informed decision to enroll in a trial (r=0.4), which may partially be explained by the absence of a tangible trial opportunity. While we did not assess specifically whether patients in our study were offered a trial, there was a substantial trial menu at NM with limited screen failures and decent trial accrual over the time frame of our study. This underscores the importance not only of patient-focused strategies to increase educational and attitudinal resources, but also a need to focus on research-site optimization that includes opening of multiple trials in various settings, systematic pre-screening of patients, and eligibility criteria that are inclusive and rational.5
The patient population with metastatic disease demonstrated more positive attitudes towards enrolling in clinical trials. This cohort accrued well to clinical trials, with 21 of 24 trials enrolling specifically patients with metastatic disease. Of the patients who responded to our survey, patients with previous trial exposure were enriched for patients with metastatic disease (53.3% metastatic among previous trial exposure versus 26.8% metastatic overall). These observations are likely reflective of the need for novel therapies in this disease setting. At the same time, approximately 25% of patients with localized soft tissue sarcoma will develop distant metastatic disease after successful treatment of their primary tumor, which increases to 40% to 50% in larger and higher-grade tumors.18 Three of 24 trials were open for patients with non-metastatic disease, of which one managed to accrue patients. Patients with non-metastatic disease had more negative attitudes towards trial enrollment. These disproportionate findings suggest a need for interventions to increase patient awareness and attitudes towards trial enrollment among this patient population and the importance of research-site optimization for trial opportunities across disease states.
Molecular profiling of tumors and biomarker identification has become a critical component of further characterizing cancer subtypes. In our study, a majority (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their molecular profile. Molecular data on 5,749 bone and soft tissue sarcomas suggested that 9.5% of tumors demonstrate a “targetable result,” defined as a new molecular finding for which there is an FDA-approved drug for malignancies other than sarcoma (eg, BRAF V600E, Her2, etc.),19 suggesting an overestimation in our patient cohort of the likelihood of benefit of molecular profiling. These results highlight that the growing use of molecular profiling has increased the need for educational and supportive resources to help patients understand the utility of molecular profiling and aid in shared decision-making surrounding the results.
At the same time, the importance of identifying a targetable mutation in patients with sarcoma cannot be understated. As a recent example of this paradigm, tumors that harbor fusions with the neurotrophic receptor tyrosine kinase 1, 2, or 3 (NTRK1, 2, and 3) have a high response rate (~75%) to drugs that target these fusions, such as larotrectinib and entrectinib.13 Molecular profiling and identification of predictive biomarkers in small patient subsets has led to great challenges in trial design and research-site optimization. Novel designs that incorporate molecular profiling,20 such as the Lung- MAP trial21 and NCI’s Molecular Analysis for Therapy Choice (MATCH) trial,22 are emerging to identify new therapies for small patient subsets. As a rare and increasingly heterogeneous cancer, sarcoma represents a paradigm to provide insight into optimizing patient perceptions and research enterprises to maximize clinical trial enrollment.
Some limitations of our study include a homogeneous and selected patient population that was predominantly Caucasian and highly educated. Therefore, these findings should not be extrapolated to other populations with barriers to trial accrual, such as lower socioeconomic or minority populations. The low response rate and failure of some to complete the survey may have introduced some bias. Additionally, our data include self-reported outcomes, which could have affected our results. Finally, the limited number of patients who had undergone or heard of molecular profiling limited our ability to draw definitive conclusions, and should be assessed in larger patient cohorts.
While our paper addresses a unique population—the sarcoma patient—similar themes and issues pertain to all oncology patients. A recent review was published in the American Society of Clinical Oncology Educational Book23 looking at methods to overcome barriers to clinical trial enrollment. Their paper clearly illustrates mechanisms to assist with overcoming financial burdens associated with cancer clinical trials, overcoming barriers as they relate to patient and clinician difficulty in coping with the uncertainty inherent in clinical trial participation, and highlight the role of a patient navigator in clinical trial participation.
Conclusions
Interventions aimed at increasing awareness, knowledge, and attitudes towards clinical trials among sarcoma patients may lead to increased trial enrollment and greater progress in cancer treatment in this population. In addition to patient- focused interventions, thoughtful and strategic clinical trial designs that allow for the development of biomarker- driven therapeutics, while at the same time optimizing patient accrual rates, should be developed. Evaluation of barriers to clinical trial enrollment and molecular profiling of tumors among bone and soft tissue sarcoma patients at an academic center can serve as a paradigm to overcome barriers to enrollment in the era of an increasingly heterogeneous cancer population. TSJ
Introduction
The development of new cancer therapies relies on the successful development and completion of clinical trials. While clinical trials have led to significant improvements in cancer treatment, the success is dependent upon patient enrollment and participation. Unfortunately, fewer than 5% of adult patients enroll in trials.1-3 This represents a significant barrier to the development and approval of new cancer treatments. Reasons for low accrual into trials are multifactorial, but include structural barriers (eg, clinic access), clinical barriers (eg, eligibility criteria), and physician and patient attitudes towards trial enrollment.4,5 One study at the University of California Davis Cancer Center reported 49% of patients declined participation despite meeting eligibility criteria,3,6 suggesting that psychosocial barriers such as knowledge of trials and attitudes towards clinical research are a major impediment to accrual.7-9
Bone and soft tissue sarcoma represent a heterogeneous group of tumors of mesenchymal origin that are an important cause of morbidity and mortality. Local disease is often treated with a multidisciplinary approach including surgery, radiation, and systemic therapy. Metastatic disease is predominantly treated palliatively with systemic therapy.10 Given its rarity and heterogeneity, trial accrual is of particular importance in sarcoma and often requires multiple sites to enroll adequate numbers of patients. While sarcoma represents <1% of adult malignancies overall, it constitutes ~15% of malignancies in the adolescent and young adult (AYA) population (15- 39 years old).11,12 Sarcoma represents a patient population in which low trial accrual has been correlated with lack of progress in cancer-related outcomes in both the adult and AYA populations.13 The reasons for low accrual rates among patients with sarcoma are poorly understood.
Sarcomas represent a molecularly and biologically heterogeneous group of malignancies with over 100 different subtypes.12 As a result, there has been significant interest in performing molecular profiling, or genetic sequencing, to identify “targetable” mutations. Targetable mutations refer to a specific genetic change identified within the tumor molecular profile for which there is a specific drug that may demonstrate activity against a particular tumor. Given the widespread utilization of this technology in sarcoma, identifying and understanding patient perceptions with regard to molecular profiling is critically important in this disease.14
In this study, we use a cross-sectional design to describe patient perceptions of trial enrollment among patients with bone and soft tissue sarcoma through validated measures, including attitudes towards clinical trials, knowledge of clinical trials, and perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about clinical trial participation, receptivity to learning more about clinical trials, and willingness to participate in clinical trials.6 In addition, we describe this patient cohort’s perceptions of molecular profiling, as current and future trials are increasingly driven by molecular or other biomarkers.
Methods
This was a cross-sectional electronic survey study of patients with bone and soft tissue sarcoma treated at Northwestern Medicine (NM) over a 5-year period. NM Enterprise Data Warehouse (NMEDW) is a single, comprehensive, and integrated repository of all clinical and research data sources within NM. The study was approved by the Northwestern University Institutional Review Board.
Survey
The investigators designed a self-administered, online survey, which was built using Research Electronic Data Capture (REDCap). The survey consisted of three sections that were answered using skip logic—a custom path through the survey that varied based on patients’ answers: (1) Patient demographic information and trial perceptions (answered by all patients); (2) Thoughts about molecular profiling (answered by patients who answered “yes” to the question, “Have you heard about molecular profiling of tumors?”); and (3) Considerations to undergo molecular profiling (answered by patients who answered “yes” to the question, “Have you undergone profiling of your cancer?”).
Clinical trial perceptions included questions assessing (1) patient knowledge about trials; (2) patient attitudes toward trials; (3) perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about trial participation; (4) receptivity to learning more about trials; and (5) willingness to participate in trials. These outcome measures had been previously developed and pilot tested for reliability and validity (TABLE 1).6
Thoughts about molecular profiling of tumors were assessed using nine items (TABLE 1). Of these, items assessing potential benefit or harm of molecular profiling were assessed using a 7-step Likert scale. Items assessing maximal benefit or harm of therapy, importance of quality vs length of life, and concern about the cost of molecular testing were assessed using a 5-step Likert scale. The study team developed and piloted these questions because there is no validated survey assessing these domains.
Considerations to undergo molecular profiling were assessed using 17 items. Items were in response to the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” Responses were assessed using a 5-point Likert scale.
Data Collection
Patients 18 years and older evaluated at NM between November 20, 2012, and November 20, 2017, with a diagnosis of sarcoma were identified by query of the NMEDW by ICD-10 codes (C40, C41.9, C44.99, C45-49, C55, C71.9, D48, D49.9, and M12.20) or equivalent ICD-9 codes. Patients were subsequently excluded if they did not have a diagnosis of bone or soft tissue sarcoma, no e-mail address listed, had died, or had not been evaluated at an NM clinic in the previous 5 years. Patients with a diagnosis of gastrointestinal stromal tumor and Kaposi’s sarcoma were also excluded.
A personalized contact e-mail was sent to patients containing an explanation of the survey and an internet link to the electronic survey through REDCap from January 2018 to March 2018. If patients did not respond to the survey, two follow-up reminder e-mails were sent 2 and 4 days following the initial survey. The link was protected so that each patient could complete the survey only once. Responses were collected through the REDCap platform. Patients read and signed an electronic consent form prior to completing the survey.
Upon completion of the survey, patients were offered a $50 VISA gift card as compensation, with an option to donate their compensation to the Robert H. Lurie Comprehensive Cancer Center Sarcoma Research Fund.
Over the described survey period, open clinical trials for patients with bone and soft tissue sarcoma available at NM were evaluated. The number of patients screened and accrued to each trial were recorded.
Statistical analysis
Responses were separated from the personal data for complete anonymization. Descriptive statistical analysis was performed for demographics and disease variables and were summarized using frequencies and percentages. Median and range were used for age. Correlations between continuous variables were analyzed using Spearman correlations. Scores were compared between subgroups using the Mann-Whitney test. Descriptive statistics for knowledge, attitude, and ability scores include means and 95% confidence intervals. Correlations were interpreted as small (r=0.10), medium (r=0.30), or large (r=0.50).15 Statistical significance was indicated when P<0.05.
Results
Patients
Seven hundred fifty patients were eligible to participate in the survey and received the initial and two follow-up e-mails. Twenty e-mailed surveys bounced back. Three hundred nine patients opened the initial e-mail and 283 patients (37.7% of total and 91.6% of opened) completed at least a portion of the survey, with 182 patients completing the entire survey (FIGURE 1). Data for analysis were used from patients who completed at least a portion of the survey.
Baseline characteristics of patients who responded can be seen in TABLE 2. Patients had a median age of 56, the majority were female (59.4%), white (88.2%), and most had college or university graduate degrees or higher educational level (69.0%). Patients had various different histological subtypes, with the most common being liposarcoma (16.5%) and leiomyosarcoma (16.0%). Slightly more than a quarter (26.8%) of patients had metastatic disease, and 84.2% had never been enrolled in a clinical trial. Previous treatments included surgery (91.1%), radiation (53.2%), and chemotherapy (51.6%). Prior to completing the survey, 85.4% reported being receptive to a cancer clinical trial, while 60.7% of patients reported willingness to participate in a clinical trial.
Knowledge, attitudes, and perceived ability
A statistically significant correlation was observed between greater knowledge of trials and more positive attitudes towards trials (P<0.001; r=0.5, FIGURE 2A). In relating patient attitudes with perceived ability, again a significant correlation was seen (P<0.001; r=0.4, FIGURE 2B). In contrast, knowledge had a weak correlation with perceived ability (P=0.024; r=0.2, FIGURE 2C). There was no difference regarding patient knowledge, attitudes, or perceived ability by age, gender, race, or income.
Knowledge, attitudes, perceived ability, and clinical trial enrollment
Thirty patients reported clinical trial experience (either previously or currently enrolled in trials) and 160 patients were never enrolled. Of the 30 patients with trial experience, 7 reported being currently enrolled, while 23 reported previous enrollment. Of these patients, 16 had metastatic disease, while 12 had non-metastatic disease, and 2 were unsure whether or not they had metastatic disease.
Patients with previous clinical trial exposure (currently or previously enrolled in clinical trials) demonstrated significantly greater trial knowledge, with a mean knowledge score of 9.3 (CI 8.5-10.0) compared with 7.7 (CI 7.3-8.1) among patients without trial exposure (P=0.002; FIGURE 3A). Similarly, patients with trial experience also had statistically significant more positive attitudes towards trials as compared with patients with no trial experience, with a mean attitude score of 3.8 (CI 3.6-4.0) and 3.5 (CI 3.4-3.6), respectively (P=0.001; FIGURE 3B). While numerically patients with trial experience have greater perceived ability compared with patients with no trial experience, with a mean score of 4.4 (CI 4.2-4.6) and 4.2 (CI 4.1-4.3), respectively, this difference did not reach statistical significance (FIGURE 3C).
Knowledge, attitudes, perceived ability, and disease stage
An analysis was performed comparing patients with metastatic vs non-metastatic disease. It was observed that patients with metastatic disease had similar knowledge of trials compared with non-metastatic patients, with a mean knowledge score of 8.4 (CI 7.7- 9.1) and 7.9 (CI 7.5-8.4), respectively, (P=0.3; FIGURE 4A). In contrast, patients with metastatic disease had more positive attitudes compared with non-metastatic patients, with a mean score of 3.7 (CI 3.5-3.8) and 3.5 (CI 3.4-3.6), respectively, which was statistically significant (P=0.03; FIGURE 4B). There was no difference in perceived ability in metastatic vs non-metastatic patients (FIGURE 4C).
Thoughts about molecular profiling
Of the total number of patients, 46 patients had heard of molecular profiling and were presented with questions regarding their thoughts (TABLE 3). Approximately two-thirds (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their tumor molecular profile. A majority (71.7%) of patients thought that a new experimental therapy chosen based on a patient’s tumor molecular profile would have at least a 50% chance of controlling the cancer. Somewhat less than a third (30.4%) of patients thought that total cure is the maximal benefit a patient could experience as a result of a treatment on a clinical trial using a drug chosen based on molecular tests. About half (52.2%) of patients agreed with the statement, “I am concerned about the cost of the test to molecularly profile my cancer.”
Considerations to undergo molecular profiling
Eighteen patients had undergone molecular profiling of their tumor. These patients were posed the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” (TABLE 4). A majority (83.3%) of patients stated that wanting to live as long as possible was important, and 72.2% of patients stated that quality of life was important. A majority (83.3%) of patients stated that hope for a cure was an extremely or quite a bit important consideration. Helping future cancer patients was extremely or quite a bit important for 77.8% of patients, while wanting to be a part of research was not at all or of little importance in 50.0% of patients.
Clinical trials at NM
Twenty-four clinical trials were available at NM between the years 2012 and 2017 for patients with bone and soft tissue sarcoma. Of these trials, 3 of 24 were for non-metastatic patients, while the remaining 21 were open only to metastatic patients. The median number of patients screened per trial was 11 (range 0-66) and the median number of patients accrued per trial was 9 (range 0-58). Of the 24 trials, 17 were not subtype specific (13 included soft tissue sarcoma alone while 4 included both bone and soft tissue sarcoma). The remaining 7 trials were sarcoma subtype specific (eg, angiosarcoma, liposarcoma, etc). Trials available at NM during this period are included in TABLE 5. There were 318 patients screened and 262 patients accrued to sarcoma trials over this time period, with a screen failure rate of 17.6% overall.
Discussion
Our study sought to describe perceptions of clinical trial enrollment among patients with bone and soft tissue sarcoma in order to elucidate and overcome barriers to enrollment, which to the best of our knowledge had not been previously described. Using previously validated patient- reported outcomes in the literature,6 our data reveal a correlation between knowledge of trials and more positive attitudes towards trials. This underscores the importance of awareness and educational strategies in this cancer population as a whole. Interventions should focus on patient perceptions that contribute to lack of participation, such as fear of side effects, loss of control (eg, idea of placebo or randomization), logistical challenges (eg, additional time or convenient location), and cost.3,5,7,16,17 For example, patients concerned about randomization should be educated on equipoise and other ethical considerations in trial design.5 Previous research has suggested that a multimedia psychoeducational intervention was effective in improving attitudes toward trials.6 Educating patients on the essential role of trials in oncology care, as demonstrated by the vast number of new drug approvals in recent years, is an essential strategy to improve attitudes, and subsequently leads to higher patient accrual rates.
In our study, both knowledge and at titudes were increased in patients with previous trial exposure. This suggests that either patients with greater knowledge and more positive attitudes are more likely to enroll in trials, or that patients with direct trial exposure are more knowledgeable and develop more positive attitudes. Our patient population was overall receptive to learning more about clinical trials (85.4%) and willing to participate (65.8%). At the same time, our study demonstrated low to medium correlations between attitudes and perceived ability to take steps towards making an informed decision to enroll in a trial (r=0.4), which may partially be explained by the absence of a tangible trial opportunity. While we did not assess specifically whether patients in our study were offered a trial, there was a substantial trial menu at NM with limited screen failures and decent trial accrual over the time frame of our study. This underscores the importance not only of patient-focused strategies to increase educational and attitudinal resources, but also a need to focus on research-site optimization that includes opening of multiple trials in various settings, systematic pre-screening of patients, and eligibility criteria that are inclusive and rational.5
The patient population with metastatic disease demonstrated more positive attitudes towards enrolling in clinical trials. This cohort accrued well to clinical trials, with 21 of 24 trials enrolling specifically patients with metastatic disease. Of the patients who responded to our survey, patients with previous trial exposure were enriched for patients with metastatic disease (53.3% metastatic among previous trial exposure versus 26.8% metastatic overall). These observations are likely reflective of the need for novel therapies in this disease setting. At the same time, approximately 25% of patients with localized soft tissue sarcoma will develop distant metastatic disease after successful treatment of their primary tumor, which increases to 40% to 50% in larger and higher-grade tumors.18 Three of 24 trials were open for patients with non-metastatic disease, of which one managed to accrue patients. Patients with non-metastatic disease had more negative attitudes towards trial enrollment. These disproportionate findings suggest a need for interventions to increase patient awareness and attitudes towards trial enrollment among this patient population and the importance of research-site optimization for trial opportunities across disease states.
Molecular profiling of tumors and biomarker identification has become a critical component of further characterizing cancer subtypes. In our study, a majority (65.2%) thought there would be a 50% or greater likelihood of finding a targetable result in their molecular profile. Molecular data on 5,749 bone and soft tissue sarcomas suggested that 9.5% of tumors demonstrate a “targetable result,” defined as a new molecular finding for which there is an FDA-approved drug for malignancies other than sarcoma (eg, BRAF V600E, Her2, etc.),19 suggesting an overestimation in our patient cohort of the likelihood of benefit of molecular profiling. These results highlight that the growing use of molecular profiling has increased the need for educational and supportive resources to help patients understand the utility of molecular profiling and aid in shared decision-making surrounding the results.
At the same time, the importance of identifying a targetable mutation in patients with sarcoma cannot be understated. As a recent example of this paradigm, tumors that harbor fusions with the neurotrophic receptor tyrosine kinase 1, 2, or 3 (NTRK1, 2, and 3) have a high response rate (~75%) to drugs that target these fusions, such as larotrectinib and entrectinib.13 Molecular profiling and identification of predictive biomarkers in small patient subsets has led to great challenges in trial design and research-site optimization. Novel designs that incorporate molecular profiling,20 such as the Lung- MAP trial21 and NCI’s Molecular Analysis for Therapy Choice (MATCH) trial,22 are emerging to identify new therapies for small patient subsets. As a rare and increasingly heterogeneous cancer, sarcoma represents a paradigm to provide insight into optimizing patient perceptions and research enterprises to maximize clinical trial enrollment.
Some limitations of our study include a homogeneous and selected patient population that was predominantly Caucasian and highly educated. Therefore, these findings should not be extrapolated to other populations with barriers to trial accrual, such as lower socioeconomic or minority populations. The low response rate and failure of some to complete the survey may have introduced some bias. Additionally, our data include self-reported outcomes, which could have affected our results. Finally, the limited number of patients who had undergone or heard of molecular profiling limited our ability to draw definitive conclusions, and should be assessed in larger patient cohorts.
While our paper addresses a unique population—the sarcoma patient—similar themes and issues pertain to all oncology patients. A recent review was published in the American Society of Clinical Oncology Educational Book23 looking at methods to overcome barriers to clinical trial enrollment. Their paper clearly illustrates mechanisms to assist with overcoming financial burdens associated with cancer clinical trials, overcoming barriers as they relate to patient and clinician difficulty in coping with the uncertainty inherent in clinical trial participation, and highlight the role of a patient navigator in clinical trial participation.
Conclusions
Interventions aimed at increasing awareness, knowledge, and attitudes towards clinical trials among sarcoma patients may lead to increased trial enrollment and greater progress in cancer treatment in this population. In addition to patient- focused interventions, thoughtful and strategic clinical trial designs that allow for the development of biomarker- driven therapeutics, while at the same time optimizing patient accrual rates, should be developed. Evaluation of barriers to clinical trial enrollment and molecular profiling of tumors among bone and soft tissue sarcoma patients at an academic center can serve as a paradigm to overcome barriers to enrollment in the era of an increasingly heterogeneous cancer population. TSJ
1. Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106(2):426-433.
2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004; 291(22):2720-2726.
3. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728-1733.
4. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185-198.
5. Cancer Action Network American Cancer Society. Barriers to patient enrollment in therapeutic clinical trials for cancer. 2018: https:// www.fightcancer.org/policy-resources/clinical- trial-barriers#figures. Accessed March 14, 2019.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw. 2007;5(8):655-664.
8. Cox K, McGarry J. Why patients don’t take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care (Engl). 2003;12(2): 114-122.
9. Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta- analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141-148.
10. National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 4.2019). https:// www.nccn.org/professionals/physician_gls/ pdf/sarcoma.pdf. Accessed October 30, 2019.
11. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
12. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncol. 2018(2):1-4.
13. Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891-1897.
14. Gornick MC, Cobain E, Le LQ, et al. Oncologists’ use of genomic sequencing data to inform clinical management. JCO Precision Oncol. 2018(2):1-13.
15. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988.
16. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536- 542.
17. Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17(9):1180-1190.
18. Singhi EK, Moore DC, Muslimani A. Metastatic soft tissue sarcomas: a review of treatment and new pharmacotherapies. P T. 2018;43(7): 410-429.
19. Gounder MM, Ali SM, Robinson V, et al. Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J Clin Oncol. 2017;35(15_suppl):abstr 11001.
20. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62-70.
21. Steuer CE, Papadimitrakopoulou V, Herbst RS, et al. Innovative clinical trials: the LUNGMAP study. Clin Pharmacol Ther. 2015;97(5): 488-491.
22. McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. 2015;107(7).
23. Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105-114.
1. Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106(2):426-433.
2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004; 291(22):2720-2726.
3. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728-1733.
4. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185-198.
5. Cancer Action Network American Cancer Society. Barriers to patient enrollment in therapeutic clinical trials for cancer. 2018: https:// www.fightcancer.org/policy-resources/clinical- trial-barriers#figures. Accessed March 14, 2019.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw. 2007;5(8):655-664.
8. Cox K, McGarry J. Why patients don’t take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care (Engl). 2003;12(2): 114-122.
9. Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta- analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141-148.
10. National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 4.2019). https:// www.nccn.org/professionals/physician_gls/ pdf/sarcoma.pdf. Accessed October 30, 2019.
11. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
12. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncol. 2018(2):1-4.
13. Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891-1897.
14. Gornick MC, Cobain E, Le LQ, et al. Oncologists’ use of genomic sequencing data to inform clinical management. JCO Precision Oncol. 2018(2):1-13.
15. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988.
16. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536- 542.
17. Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17(9):1180-1190.
18. Singhi EK, Moore DC, Muslimani A. Metastatic soft tissue sarcomas: a review of treatment and new pharmacotherapies. P T. 2018;43(7): 410-429.
19. Gounder MM, Ali SM, Robinson V, et al. Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J Clin Oncol. 2017;35(15_suppl):abstr 11001.
20. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62-70.
21. Steuer CE, Papadimitrakopoulou V, Herbst RS, et al. Innovative clinical trials: the LUNGMAP study. Clin Pharmacol Ther. 2015;97(5): 488-491.
22. McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. 2015;107(7).
23. Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105-114.