User login
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.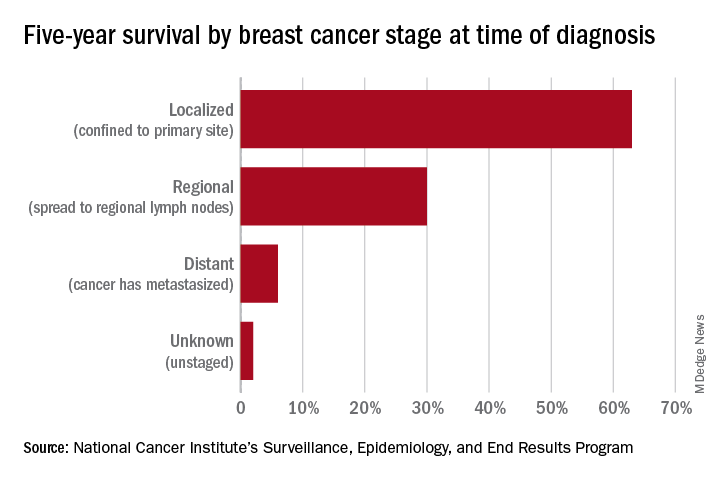
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.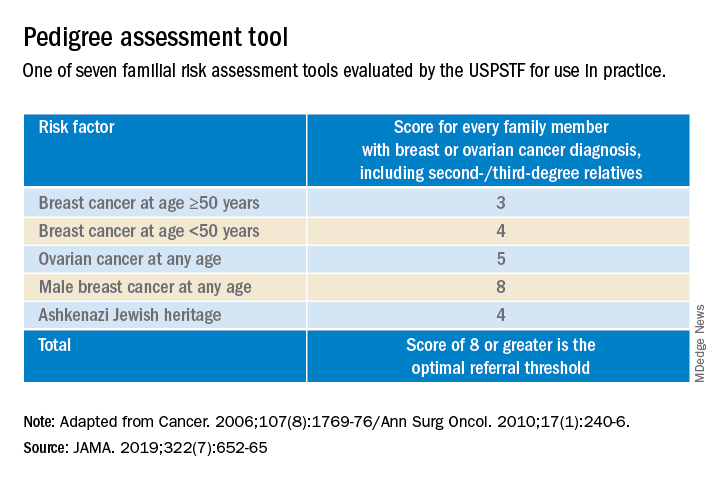
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.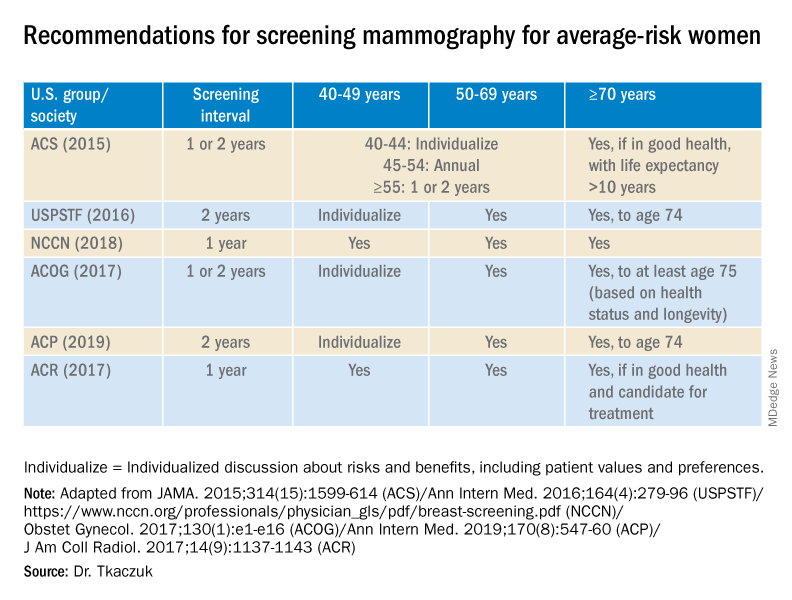
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.

