User login
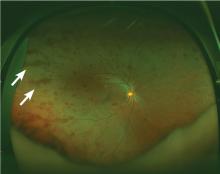
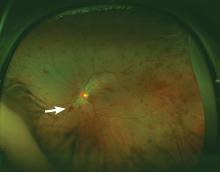
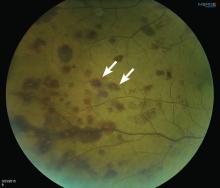
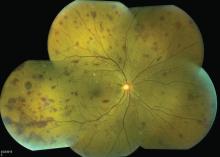
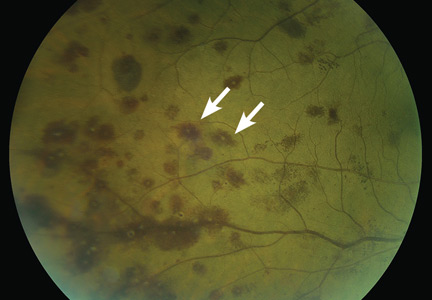
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
A 51-year-old woman with no significant medical history presented to her primary care physician because of blurred vision, increased fatigue, palpitations, and intermittent episodes of epistaxis. Vital signs were within normal limits except for a heart rate of 110 beats per minute. Physical examination revealed pale conjunctivae and bilateral white-centered retinal hemorrhages and microaneurysms
(Figures 1–4).
The results of laboratory studies:
- Hemoglobin 2.4 g/dL (reference range 12–16)
- Platelet count 78 × 109/L (150–400)
- White blood cell count 4.0 × 109/L (3.7–11.0)
- Atypical lymphocytes 18% (0.0–3.0%)
- Reticulocyte index 0.3 (0.5–2.5%)
- Gamma gap 7.0 g/dL (< 4)
- Immunoglobulin A (IgA) 5,560 mg/dL (61–356).
Bone marrow biopsy study showed complete effacement of the hematopoietic elements of normal marrow, and the diagnosis of B cell lymphoplasmacytic lymphoma was made. Serum electrophoresis showed an elevated kappa-to-lambda ratio of 4.6500 (reference range 0.2600–1.6500). B cells expressed monotypic kappa surface immunoglobulin light chains CD19, CD20, and CD22. They did not express CD5. No testing was done for the MYD88 point mutation.
ROTH SPOTS
White-centered retinal hemorrhages, or Roth spots, are the result of rupture of retinal vessels with subsequent accumulation of platelets and fibrin surrounded by blood.1 Although Roth spots are mistakenly believed to be caused only by infective endocarditis, they are seen in a variety of conditions, including leukemia, anemia, thrombocytopenia, and hypoxia. Each of these conditions has a different mechanism for vessel rupture, which can include fragility of the smooth muscle vessel wall from hypoxemia and increased hydrostatic pressure in hyperviscosity syndrome.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome and is usually associated with Waldenström macroglobulinemia, a type of lymphoplasmacytic lymphoma associated with the largest immunoglobulin, IgM. However, our patient presented with a variant of Waldenström macroglobulinemia with high levels of IgA, a small molecule that in high quantities can also cause hyperviscosity.
Immediate treatment is aimed at decreasing blood viscosity with plasmapheresis and controlling the underlying disease with chemotherapy.2 There have been cases of cancer-related Roth spots, in which the lesions disappeared after chemotherapy.3
DIFFERENTIAL DIAGNOSIS OF ROTH SPOTS
Roth spots share morphologic features with other retinal abnormalities such as “cotton wool” spots, Purtscher retinopathy, and cytomegalovirus retinitis. A thorough history and careful ophthalmologic examination can help differentiate these from Roth spots.
Cotton wool spots are a nonspecific sign of vascular insufficiency and represent an ischemic, inflammatory, or infectious condition or an embolic or neoplastic process. Most often, they represent retinopathy from poorly controlled diabetes mellitus or hypertension. On funduscopic examination, cotton wool spots are small, irregularly shaped, yellow-white plaques that may appear raised. They are often found over the posterior pole of the fundus. In contrast, Roth spots appear white or pale on a background of hemorrhage.
Purtscher retinopathy. Polygonal retinal whitening with sharp demarcation against the normal retina is pathognomonic of Purtscher retinopathy.4 Purtscher lesions may represent a traumatic or nontraumatic condition, including closed head injury, barotrauma, pancreatic disorder, connective tissue disease, fat embolism after long bone fracture, and even microangiopathic hemolytic anemia. Patients usually describe diminished visual acuity in the context of injury or other systemic illness.
Cytomegalovirus retinitis is an important consideration in the immunocompromised patient presenting with retinal hemorrhage and is the most common cause of blindness in acquired immunodeficiency syndrome (AIDS).5 It is a slowly progressive disease, initially asymptomatic and involving one eye, but in some untreated patients with AIDS it may progress to the contralateral eye. When it progresses, patients usually present with floaters or vision loss. The retina can have cotton wool spots with a more diffuse pattern of hemorrhage on the periphery and white sheathing along blood vessels, eloquently described as “frosted branch angiitis.”
Our patient’s lesions appeared more punctate, in contrast to the often fulminant hemorrhagic necrosis or perivascular white lesions surrounding retinal vessels characteristic of cytomegalovirus retinitis.
THE NEED FOR ACTION
New-onset blurred vision should prompt a comprehensive history and physical examination, as it may be secondary to a life-threatening systemic disease. A thorough funduscopic examination may provide vital information and guide management and expeditious referrals for patients with time-sensitive conditions such as cancer.
Our patient decided to undergo treatment outside our hospital and so was lost to follow-up.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.
- Ling R, James B. White-centred retinal haemorrhages (Roth spots). Postgrad Med J 1998; 74:581–582.
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29:467–471.
- Docherty SM, Reza M, Turner G, Bowles K. Resolution of Roth spots in chronic myeloid leukaemia after treatment with imatinib. Br J Haematol 2015; 170:744.
- Miguel AI, Henriques F, Azevedo LF, Loureiro AJ, Maberley DA. Systematic review of Purtscher’s and Purtscher-like retinopathies. Eye (Lond) 2013; 27:1–13.
- Kestelyn PG, Cunningham ET Jr. HIV/AIDS and blindness. Bull World Health Organ 2001; 79:208–213.