User login
CD30+ primary cutaneous lymphoproliferative disorders (pcLPDs) are the second most common cause of cutaneous T-cell lymphoma, accounting for approximately 25% to 30% of cases.1 These disorders comprise a spectrum that includes primary cutaneous anaplastic large-cell lymphoma (pcALCL); lymphomatoid papulosis (LyP); and borderline lesions, which share clinicopathologic features of both pcALCL and LyP. Lymphomatoid papulosis is characterized as chronic, recurrent, papular or papulonodular skin lesions that typically are multifocal and regress spontaneously within weeks to months, only leaving small scars with atrophy and/or hyperpigmentation.2 Cutaneous anaplastic large-cell lymphoma typically presents as solitary or grouped nodules or tumors that may undergo spontaneous partial or complete regression in approximately 25% of cases3 but often persist if not treated. Patients may have an array of lesions comprising the spectrum of CD30 pcLPDs.4
There is no curative therapy for CD30+ pcLPDs. Although active treatment is not necessary for LyP, low-dose methotrexate (MTX)(10–50 mg weekly) or phototherapy are the preferred initial suppressive therapies for symptomatic patients with scarring, facial lesions, or multiple symptomatic lesions.5 Observation with expectant follow-up is an option in pcALCL, though spontaneous regression is less likely than in LyP. For single or grouped pcALCL lesions, local radiation is the first-line therapy.6 Multifocal pcALCL lesions also can be treated with low-dose MTX,2,5 as in LyP, or local radiation to selected areas. Although local radiotherapy is considered a first-line treatment in pcALCL, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. We report the complete response of refractory pcALCL lesions to low-dose radiation while remaining on MTX weekly without any adverse effects.
Case Report
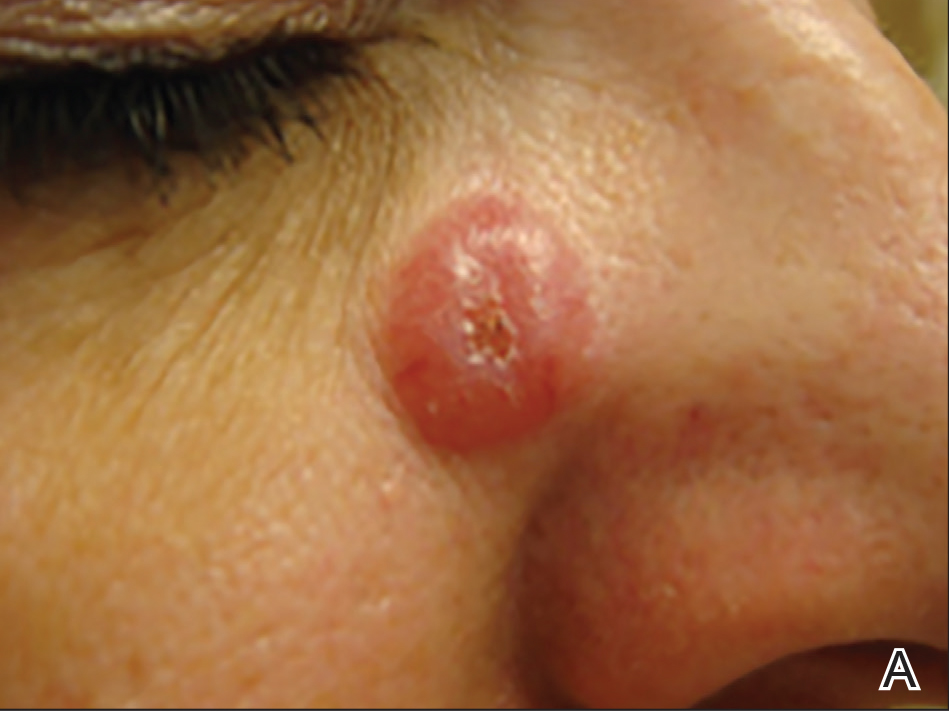
A 51-year-old woman presented with a 3-year history of CD30+ pcLPD manifesting primarily as pcALCL involving the head and neck, as well as LyP involving the head, arms, and trunk (T3N0M0). For 2 years her treatment regimen included clobetasol propionate cream 0.05% as needed for new lesions and 2 courses of standard-dose localized external beam radiation for larger pcALCL tumors on the right cheek and right side of the chin (Figure 1)(total dose for each course of treatment was 20 Gy and 36 Gy, respectively, each administered over 2–3 weeks). Because new unsightly papulonodules continued to develop on the patient’s face, she subsequently required low-dose oral MTX 30 mg once weekly for suppression of new lesions and was stable on this regimen for a year. However, she experienced an increase in LyP/pcALCL activity on the face during a 2-week break from MTX when she developed a herpes zoster infection on the right side of the forehead.
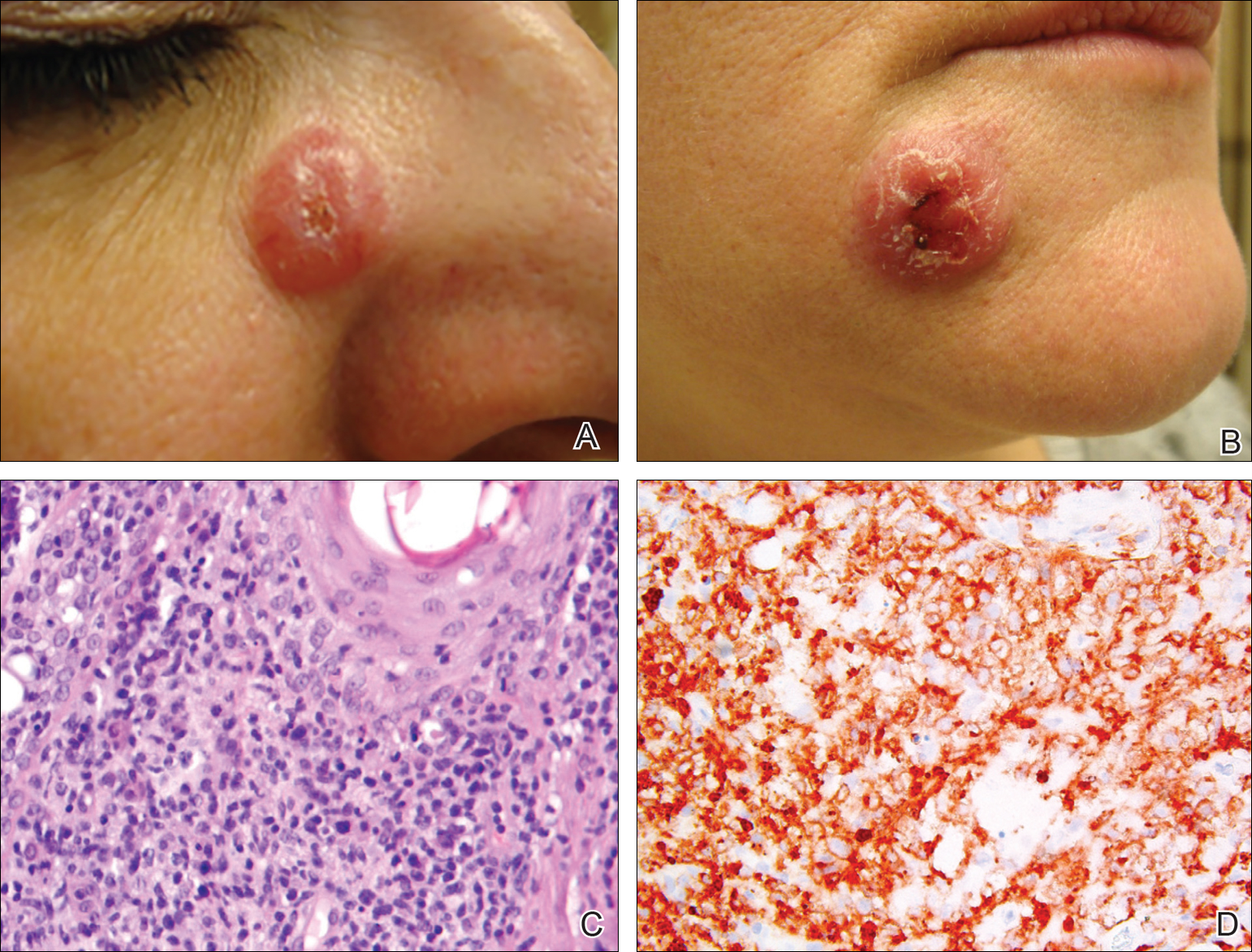
On physical examination 1 month later, 5 tiny pink papules scattered on the left eyebrow, left cheek, and left side of the chin were noted. She was advised to continue applying the clobetasol cream as needed and was restarted on MTX 10 mg once weekly. However, she developed 2 additional 1-cm nodules on the left side of the chin, neck, and shoulder. Methotrexate was increased to 30 mg once weekly over 2 weeks, which was the original dose prior to interruption, but the nodules grew to 1.5-cm in diameter. Due to their clinical appearance, the nodules were believed to be early pcALCL lesions (Figure 2A). Given the cosmetically sensitive location of the nodules, palliative radiotherapy was recommended rather than observe for possible regression. Based on a prior report by Neelis et al7 demonstrating efficacy of low-dose radiotherapy for cutaneous T-cell lymphoma and cutaneous B-cell lymphoma, we recommended starting with low radiation doses. Our patient was treated with 400 cGy twice to the left side of the chin and left side of the neck (800 cGy total at each site) while remaining on MTX 30 mg once weekly. This treatment was well tolerated without side effects and no evidence of radiation dermatitis. On follow-up examination 1 week later, the nodules had regressed and no new lesions were present (Figure 2B).
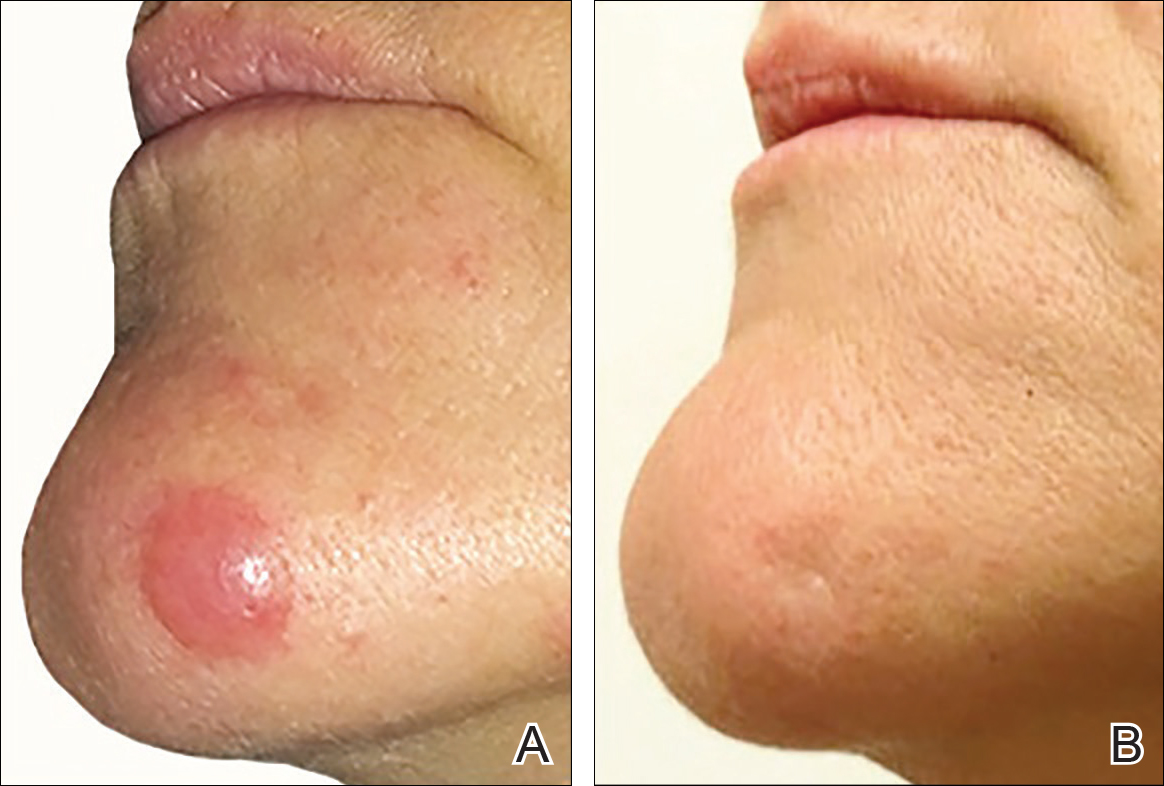
The patient has stayed on oral MTX and occasionally develops small lesions that quickly resolve with clobetasol cream. She has been followed for 3 years after radiotherapy and all 3 previously irradiated sites have remained recurrence free. Furthermore, she has not developed any new larger nodules or tumors and her MTX dose has been decreased to 15 mg once weekly.
Comment
Local radiotherapy is considered a first-line treatment of pcALCL; however, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. Although no standard dose exists for pcALCL, the National Comprehensive Cancer Network guidelines8 recommend doses of 12 to 36 Gy in mycosis fungoides/Sézary syndrome subtypes of cutaneous T-cell lymphoma, which are consistent with guidelines published by the European Society for Medical Oncology.9 High complete response rates have been demonstrated in pcALCL at doses of 34 to 44 Gy6; however, lesions tend to recur elsewhere on the skin in 36% to 41% of patients despite treatment.2,10 Lower doses of radiation therapy would provide several advantages over higher-dose therapy if a complete response could be achieved without greatly increasing the local recurrence rate. In cases of local recurrence, low-dose radiation would more easily permit retreatment of lesions compared to higher doses of radiation. Similarly, in patients with multifocal pcALCL, lower doses of radiotherapy may allow for treatment of larger skin areas while limiting potential treatment risks. Furthermore, low-dose therapy would allow for treatments to be delivered more quickly and with less inconvenience to the patient who is likely to need multiple future treatments to other areas. Low-dose radiation has been described with a favorable efficacy profile for mycosis fungoides7,11 but has not been studied in patients with CD30+ pcLPDs.
Our case is notable because the patient remained on MTX during radiation therapy. B
Conclusion
We reported the use of low-dose radiation therapy for the treatment of localized pcALCL in a patient who remained on low-dose oral MTX. Additional studies will be necessary to more fully evaluate the efficacy of using low-dose radiation both as monotherapy and in combination with MTX for pcALCL.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993;28:973-980.
- Kadin ME. The spectrum of Ki-1+ cutaneous lymphomas. Curr Probl Dermatol. 1990;19:132-143.
- Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34:470-481.
- Yu JB, McNiff JM, Lund MW, et al. Treatment of primary cutaneous CD30+ anaplastic large-cell lymphoma with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:1542-1545.
- Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy B-cell and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74:154-158.
- National Comprehensive Cancer Network. CD30 lymphoproliferative disorders section in non-Hodgkin’s lymphoma (Version 3.2016). http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed September 26, 2016.
- Willemze R, Hodak E, Zinzani PL, et al; ESMO Guidelines Working Group. Primary cutaneous lymphomas: EMSO clinical practice guidelines for diagnosis, treatment, and follow-up [published online July 17, 2013]. Ann Onc. 2013;24(suppl 6):vi149-vi154.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Harrison C, Young J, Navi D, et al. Revisiting low dose total skin electron beam radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2011;81:651-657.
- Jaffe N, Farber S, Traggis D, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31:1367-1373.
- Rosen G, Tefft M, Martinez A, et al. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35:622-630.
- Kim YH, Aye MS, Fayos JV. Radiation necrosis of the scalp: a complication of cranial irradiation and methotrexate. Radiology. 1977;124:813-814.
CD30+ primary cutaneous lymphoproliferative disorders (pcLPDs) are the second most common cause of cutaneous T-cell lymphoma, accounting for approximately 25% to 30% of cases.1 These disorders comprise a spectrum that includes primary cutaneous anaplastic large-cell lymphoma (pcALCL); lymphomatoid papulosis (LyP); and borderline lesions, which share clinicopathologic features of both pcALCL and LyP. Lymphomatoid papulosis is characterized as chronic, recurrent, papular or papulonodular skin lesions that typically are multifocal and regress spontaneously within weeks to months, only leaving small scars with atrophy and/or hyperpigmentation.2 Cutaneous anaplastic large-cell lymphoma typically presents as solitary or grouped nodules or tumors that may undergo spontaneous partial or complete regression in approximately 25% of cases3 but often persist if not treated. Patients may have an array of lesions comprising the spectrum of CD30 pcLPDs.4
There is no curative therapy for CD30+ pcLPDs. Although active treatment is not necessary for LyP, low-dose methotrexate (MTX)(10–50 mg weekly) or phototherapy are the preferred initial suppressive therapies for symptomatic patients with scarring, facial lesions, or multiple symptomatic lesions.5 Observation with expectant follow-up is an option in pcALCL, though spontaneous regression is less likely than in LyP. For single or grouped pcALCL lesions, local radiation is the first-line therapy.6 Multifocal pcALCL lesions also can be treated with low-dose MTX,2,5 as in LyP, or local radiation to selected areas. Although local radiotherapy is considered a first-line treatment in pcALCL, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. We report the complete response of refractory pcALCL lesions to low-dose radiation while remaining on MTX weekly without any adverse effects.
Case Report
A 51-year-old woman presented with a 3-year history of CD30+ pcLPD manifesting primarily as pcALCL involving the head and neck, as well as LyP involving the head, arms, and trunk (T3N0M0). For 2 years her treatment regimen included clobetasol propionate cream 0.05% as needed for new lesions and 2 courses of standard-dose localized external beam radiation for larger pcALCL tumors on the right cheek and right side of the chin (Figure 1)(total dose for each course of treatment was 20 Gy and 36 Gy, respectively, each administered over 2–3 weeks). Because new unsightly papulonodules continued to develop on the patient’s face, she subsequently required low-dose oral MTX 30 mg once weekly for suppression of new lesions and was stable on this regimen for a year. However, she experienced an increase in LyP/pcALCL activity on the face during a 2-week break from MTX when she developed a herpes zoster infection on the right side of the forehead.

On physical examination 1 month later, 5 tiny pink papules scattered on the left eyebrow, left cheek, and left side of the chin were noted. She was advised to continue applying the clobetasol cream as needed and was restarted on MTX 10 mg once weekly. However, she developed 2 additional 1-cm nodules on the left side of the chin, neck, and shoulder. Methotrexate was increased to 30 mg once weekly over 2 weeks, which was the original dose prior to interruption, but the nodules grew to 1.5-cm in diameter. Due to their clinical appearance, the nodules were believed to be early pcALCL lesions (Figure 2A). Given the cosmetically sensitive location of the nodules, palliative radiotherapy was recommended rather than observe for possible regression. Based on a prior report by Neelis et al7 demonstrating efficacy of low-dose radiotherapy for cutaneous T-cell lymphoma and cutaneous B-cell lymphoma, we recommended starting with low radiation doses. Our patient was treated with 400 cGy twice to the left side of the chin and left side of the neck (800 cGy total at each site) while remaining on MTX 30 mg once weekly. This treatment was well tolerated without side effects and no evidence of radiation dermatitis. On follow-up examination 1 week later, the nodules had regressed and no new lesions were present (Figure 2B).

The patient has stayed on oral MTX and occasionally develops small lesions that quickly resolve with clobetasol cream. She has been followed for 3 years after radiotherapy and all 3 previously irradiated sites have remained recurrence free. Furthermore, she has not developed any new larger nodules or tumors and her MTX dose has been decreased to 15 mg once weekly.
Comment
Local radiotherapy is considered a first-line treatment of pcALCL; however, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. Although no standard dose exists for pcALCL, the National Comprehensive Cancer Network guidelines8 recommend doses of 12 to 36 Gy in mycosis fungoides/Sézary syndrome subtypes of cutaneous T-cell lymphoma, which are consistent with guidelines published by the European Society for Medical Oncology.9 High complete response rates have been demonstrated in pcALCL at doses of 34 to 44 Gy6; however, lesions tend to recur elsewhere on the skin in 36% to 41% of patients despite treatment.2,10 Lower doses of radiation therapy would provide several advantages over higher-dose therapy if a complete response could be achieved without greatly increasing the local recurrence rate. In cases of local recurrence, low-dose radiation would more easily permit retreatment of lesions compared to higher doses of radiation. Similarly, in patients with multifocal pcALCL, lower doses of radiotherapy may allow for treatment of larger skin areas while limiting potential treatment risks. Furthermore, low-dose therapy would allow for treatments to be delivered more quickly and with less inconvenience to the patient who is likely to need multiple future treatments to other areas. Low-dose radiation has been described with a favorable efficacy profile for mycosis fungoides7,11 but has not been studied in patients with CD30+ pcLPDs.
Our case is notable because the patient remained on MTX during radiation therapy. B
Conclusion
We reported the use of low-dose radiation therapy for the treatment of localized pcALCL in a patient who remained on low-dose oral MTX. Additional studies will be necessary to more fully evaluate the efficacy of using low-dose radiation both as monotherapy and in combination with MTX for pcALCL.
CD30+ primary cutaneous lymphoproliferative disorders (pcLPDs) are the second most common cause of cutaneous T-cell lymphoma, accounting for approximately 25% to 30% of cases.1 These disorders comprise a spectrum that includes primary cutaneous anaplastic large-cell lymphoma (pcALCL); lymphomatoid papulosis (LyP); and borderline lesions, which share clinicopathologic features of both pcALCL and LyP. Lymphomatoid papulosis is characterized as chronic, recurrent, papular or papulonodular skin lesions that typically are multifocal and regress spontaneously within weeks to months, only leaving small scars with atrophy and/or hyperpigmentation.2 Cutaneous anaplastic large-cell lymphoma typically presents as solitary or grouped nodules or tumors that may undergo spontaneous partial or complete regression in approximately 25% of cases3 but often persist if not treated. Patients may have an array of lesions comprising the spectrum of CD30 pcLPDs.4
There is no curative therapy for CD30+ pcLPDs. Although active treatment is not necessary for LyP, low-dose methotrexate (MTX)(10–50 mg weekly) or phototherapy are the preferred initial suppressive therapies for symptomatic patients with scarring, facial lesions, or multiple symptomatic lesions.5 Observation with expectant follow-up is an option in pcALCL, though spontaneous regression is less likely than in LyP. For single or grouped pcALCL lesions, local radiation is the first-line therapy.6 Multifocal pcALCL lesions also can be treated with low-dose MTX,2,5 as in LyP, or local radiation to selected areas. Although local radiotherapy is considered a first-line treatment in pcALCL, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. We report the complete response of refractory pcALCL lesions to low-dose radiation while remaining on MTX weekly without any adverse effects.
Case Report
A 51-year-old woman presented with a 3-year history of CD30+ pcLPD manifesting primarily as pcALCL involving the head and neck, as well as LyP involving the head, arms, and trunk (T3N0M0). For 2 years her treatment regimen included clobetasol propionate cream 0.05% as needed for new lesions and 2 courses of standard-dose localized external beam radiation for larger pcALCL tumors on the right cheek and right side of the chin (Figure 1)(total dose for each course of treatment was 20 Gy and 36 Gy, respectively, each administered over 2–3 weeks). Because new unsightly papulonodules continued to develop on the patient’s face, she subsequently required low-dose oral MTX 30 mg once weekly for suppression of new lesions and was stable on this regimen for a year. However, she experienced an increase in LyP/pcALCL activity on the face during a 2-week break from MTX when she developed a herpes zoster infection on the right side of the forehead.

On physical examination 1 month later, 5 tiny pink papules scattered on the left eyebrow, left cheek, and left side of the chin were noted. She was advised to continue applying the clobetasol cream as needed and was restarted on MTX 10 mg once weekly. However, she developed 2 additional 1-cm nodules on the left side of the chin, neck, and shoulder. Methotrexate was increased to 30 mg once weekly over 2 weeks, which was the original dose prior to interruption, but the nodules grew to 1.5-cm in diameter. Due to their clinical appearance, the nodules were believed to be early pcALCL lesions (Figure 2A). Given the cosmetically sensitive location of the nodules, palliative radiotherapy was recommended rather than observe for possible regression. Based on a prior report by Neelis et al7 demonstrating efficacy of low-dose radiotherapy for cutaneous T-cell lymphoma and cutaneous B-cell lymphoma, we recommended starting with low radiation doses. Our patient was treated with 400 cGy twice to the left side of the chin and left side of the neck (800 cGy total at each site) while remaining on MTX 30 mg once weekly. This treatment was well tolerated without side effects and no evidence of radiation dermatitis. On follow-up examination 1 week later, the nodules had regressed and no new lesions were present (Figure 2B).

The patient has stayed on oral MTX and occasionally develops small lesions that quickly resolve with clobetasol cream. She has been followed for 3 years after radiotherapy and all 3 previously irradiated sites have remained recurrence free. Furthermore, she has not developed any new larger nodules or tumors and her MTX dose has been decreased to 15 mg once weekly.
Comment
Local radiotherapy is considered a first-line treatment of pcALCL; however, there is limited evidence on its clinical efficacy as well as the optimal dose and technique. Although no standard dose exists for pcALCL, the National Comprehensive Cancer Network guidelines8 recommend doses of 12 to 36 Gy in mycosis fungoides/Sézary syndrome subtypes of cutaneous T-cell lymphoma, which are consistent with guidelines published by the European Society for Medical Oncology.9 High complete response rates have been demonstrated in pcALCL at doses of 34 to 44 Gy6; however, lesions tend to recur elsewhere on the skin in 36% to 41% of patients despite treatment.2,10 Lower doses of radiation therapy would provide several advantages over higher-dose therapy if a complete response could be achieved without greatly increasing the local recurrence rate. In cases of local recurrence, low-dose radiation would more easily permit retreatment of lesions compared to higher doses of radiation. Similarly, in patients with multifocal pcALCL, lower doses of radiotherapy may allow for treatment of larger skin areas while limiting potential treatment risks. Furthermore, low-dose therapy would allow for treatments to be delivered more quickly and with less inconvenience to the patient who is likely to need multiple future treatments to other areas. Low-dose radiation has been described with a favorable efficacy profile for mycosis fungoides7,11 but has not been studied in patients with CD30+ pcLPDs.
Our case is notable because the patient remained on MTX during radiation therapy. B
Conclusion
We reported the use of low-dose radiation therapy for the treatment of localized pcALCL in a patient who remained on low-dose oral MTX. Additional studies will be necessary to more fully evaluate the efficacy of using low-dose radiation both as monotherapy and in combination with MTX for pcALCL.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993;28:973-980.
- Kadin ME. The spectrum of Ki-1+ cutaneous lymphomas. Curr Probl Dermatol. 1990;19:132-143.
- Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34:470-481.
- Yu JB, McNiff JM, Lund MW, et al. Treatment of primary cutaneous CD30+ anaplastic large-cell lymphoma with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:1542-1545.
- Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy B-cell and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74:154-158.
- National Comprehensive Cancer Network. CD30 lymphoproliferative disorders section in non-Hodgkin’s lymphoma (Version 3.2016). http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed September 26, 2016.
- Willemze R, Hodak E, Zinzani PL, et al; ESMO Guidelines Working Group. Primary cutaneous lymphomas: EMSO clinical practice guidelines for diagnosis, treatment, and follow-up [published online July 17, 2013]. Ann Onc. 2013;24(suppl 6):vi149-vi154.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Harrison C, Young J, Navi D, et al. Revisiting low dose total skin electron beam radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2011;81:651-657.
- Jaffe N, Farber S, Traggis D, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31:1367-1373.
- Rosen G, Tefft M, Martinez A, et al. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35:622-630.
- Kim YH, Aye MS, Fayos JV. Radiation necrosis of the scalp: a complication of cranial irradiation and methotrexate. Radiology. 1977;124:813-814.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993;28:973-980.
- Kadin ME. The spectrum of Ki-1+ cutaneous lymphomas. Curr Probl Dermatol. 1990;19:132-143.
- Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34:470-481.
- Yu JB, McNiff JM, Lund MW, et al. Treatment of primary cutaneous CD30+ anaplastic large-cell lymphoma with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:1542-1545.
- Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy B-cell and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74:154-158.
- National Comprehensive Cancer Network. CD30 lymphoproliferative disorders section in non-Hodgkin’s lymphoma (Version 3.2016). http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed September 26, 2016.
- Willemze R, Hodak E, Zinzani PL, et al; ESMO Guidelines Working Group. Primary cutaneous lymphomas: EMSO clinical practice guidelines for diagnosis, treatment, and follow-up [published online July 17, 2013]. Ann Onc. 2013;24(suppl 6):vi149-vi154.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Harrison C, Young J, Navi D, et al. Revisiting low dose total skin electron beam radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2011;81:651-657.
- Jaffe N, Farber S, Traggis D, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31:1367-1373.
- Rosen G, Tefft M, Martinez A, et al. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35:622-630.
- Kim YH, Aye MS, Fayos JV. Radiation necrosis of the scalp: a complication of cranial irradiation and methotrexate. Radiology. 1977;124:813-814.
Practice Points
- Cutaneous T-cell lymphoma tumors such as primary cutaneous anaplastic large-cell lymphoma can respond to low-dose radiation therapy, which enables future retreatment of sensitive sites.
- Low-dose radiation therapy requires a shorter course of therapy than traditional dosing, which is more convenient and less costly.