User login
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.
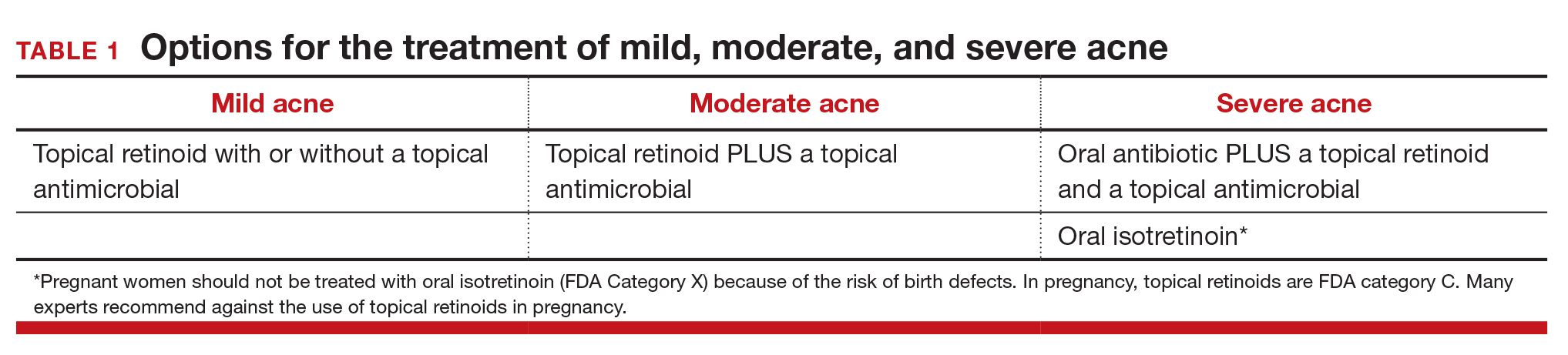
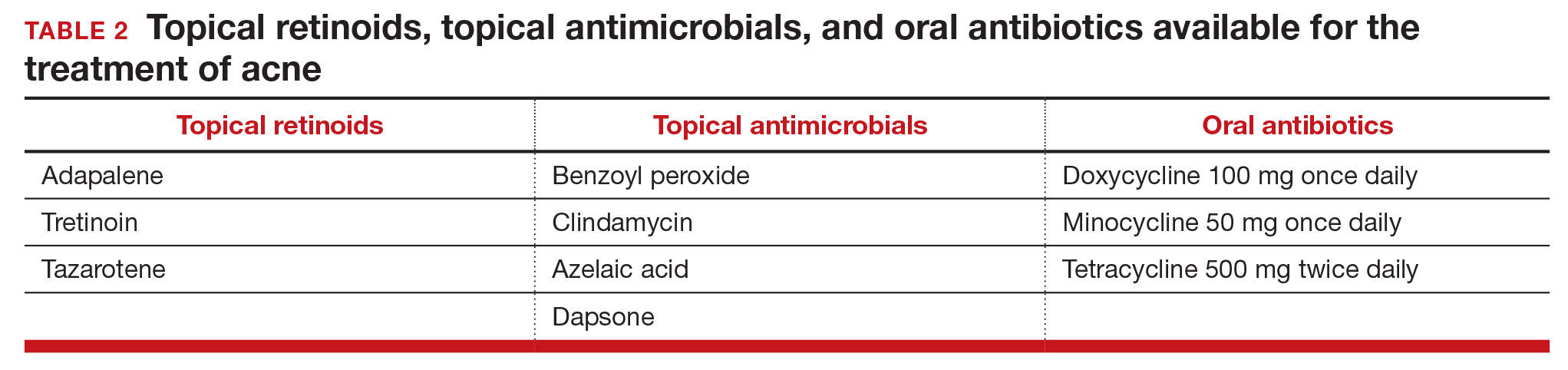
Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.


