User login
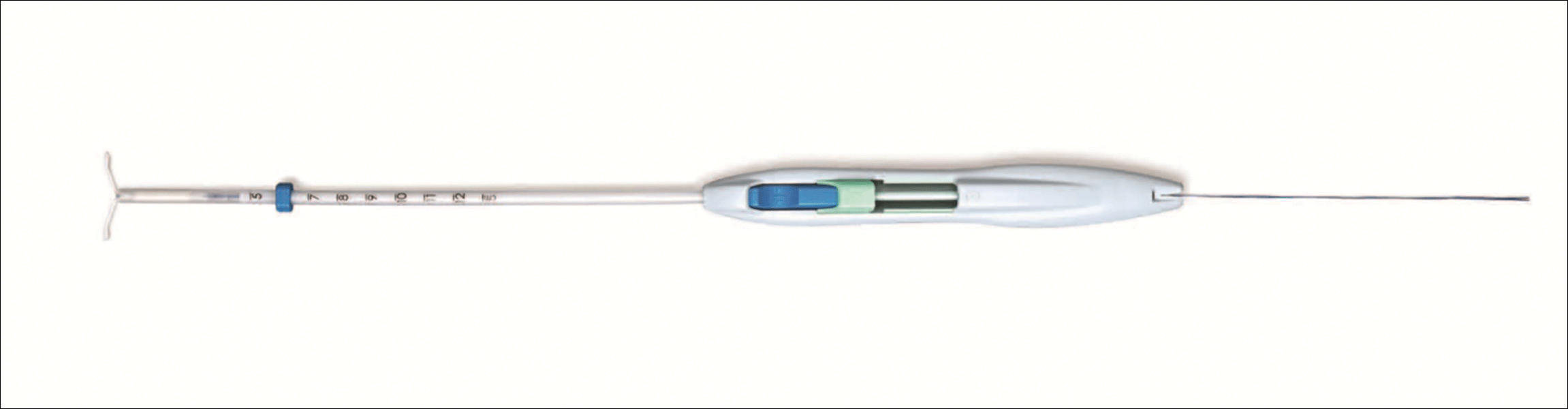
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
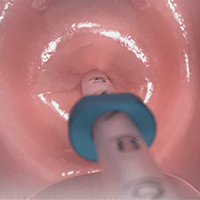
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
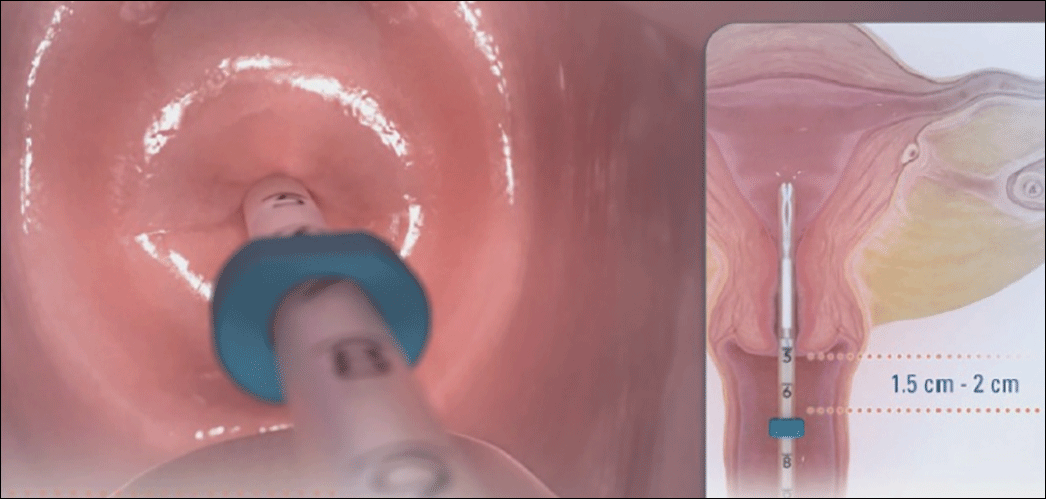 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix 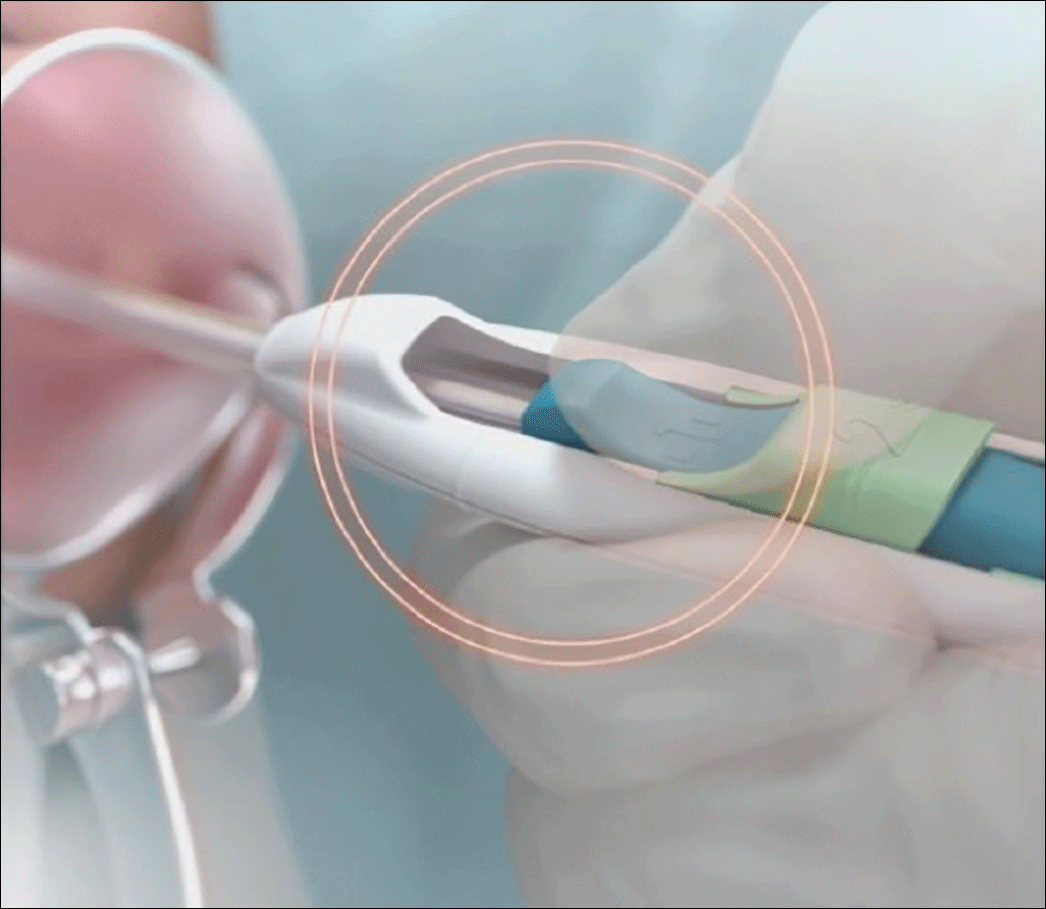 Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
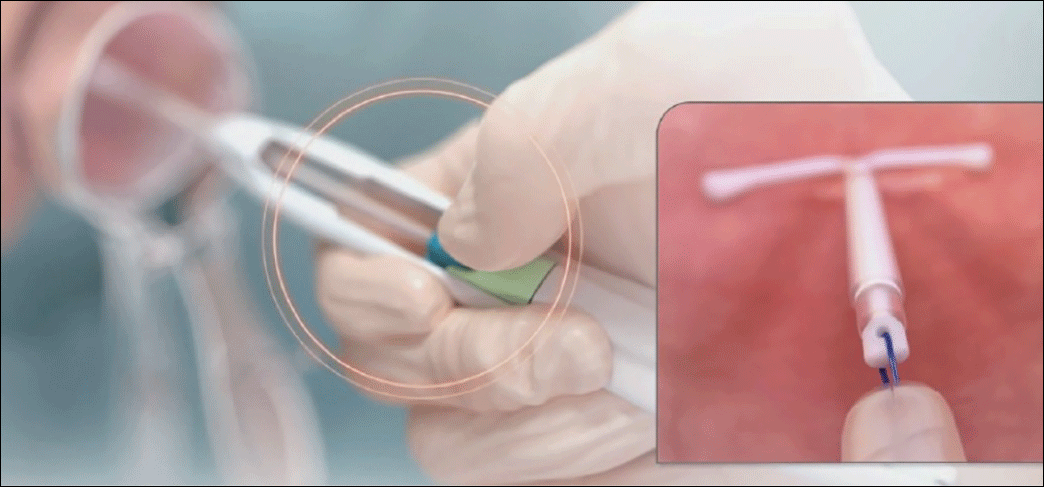 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
In this article