User login
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
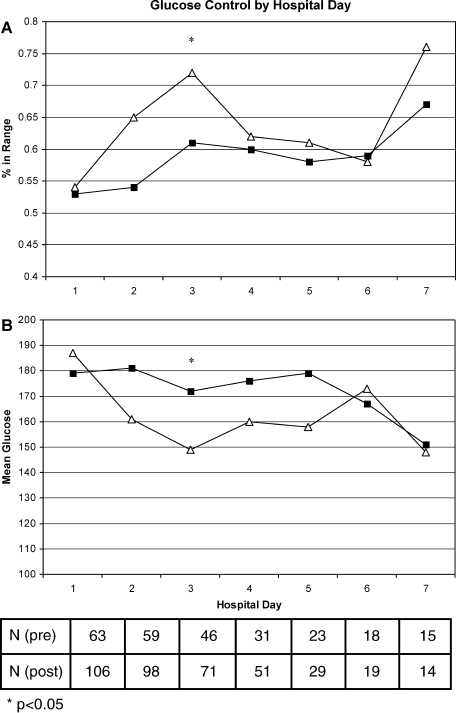
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.
DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
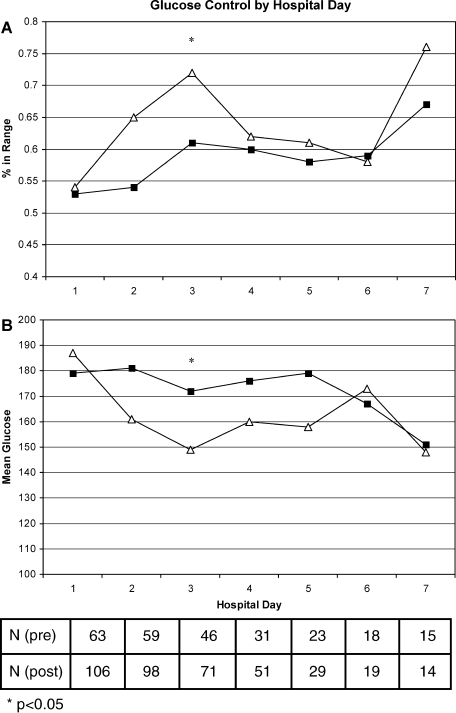
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Copyright © 2009 Society of Hospital Medicine