User login
Patients often ask primary care physicians and cardiologists about the measurement of biomarkers for cardiovascular disease and about the efficacy of preventive measures.
Although studies have shown that elevated homocysteine is a risk factor for cardiovascular and peripheral arterial disease1–3 and that supplementation with folic acid, vitamin B6, and vitamin B12 lowers homocysteine levels,4,5 it is unclear whether such supplementation prevents cardiovascular events. As a result, there is no consensus about whose homocysteine levels should be measured and who, if anyone, should receive homocysteine-lowering therapies.
The aim of this paper is to examine whether the evidence is sufficient to recommend homocysteine testing to guide the prevention and treatment of cardiovascular disease, or to recommend using folic acid, vitamin B6, and vitamin B12 for primary or secondary prevention of cardiovascular disease.
HISTORY OF HOMOCYSTEINE AS A RISK MARKER
Homocysteine is an amino acid formed from the metabolism of methionine, an essential amino acid derived from dietary protein. Although homocysteine was first isolated by Butz and du Vigneaud in 1932,6 it was not until 1964 that Gibson et al7 reported that patients with homocystinuria (more about this below) had vascular anomalies and arterial thrombosis. In 1969, McCully8 made the connection between elevated homocysteine levels and the risk of atherosclerosis.
Several possible mechanisms for the association between homocysteine and atherosclerosis have been demonstrated in experimental models. These include stimulation of smooth muscle growth, reduction in endothelial cell growth, impaired endothelial cell relaxation, decreased synthesis of high-density lipoprotein, promotion of autoimmune response, and accumulation of inflammatory monocytes in atherosclerotic plaques.3,9,10
In view of these findings, researchers have been evaluating whether homocysteine-lowering therapies decrease the risk of cardiovascular disease.
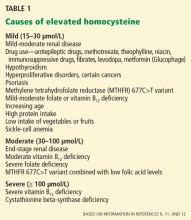
CAUSES OF ELEVATED PLASMA HOMOCYSTEINE
Vitamin deficiencies. Vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin), and folic acid are cofactors required for homocysteine metabolism, and deficiency in any or all of these leads to disruption of the relevant metabolic pathways.
Renal impairment. A low glomerular filtration rate has also been correlated with an elevated plasma homocysteine concentration. This makes sense, since the kidneys perform up to 70% of the clearance of homocysteine, although a cause-and-effect relationship is unclear.13
Inborn errors of homocysteine metabolism.Homocystinuria, ie, an abnormal elevation of homocysteine in the urine, is caused by several autosomal recessive disorders. People with these genetic variations have extremely high homocysteine levels.
A deficiency in the enzyme cystathionine beta-synthase is quite rare (the incidence in newborn babies has been found to be 1 in 344,000 worldwide and 1 in 65,000 in Ireland and Australia14), but leads to homocysteine levels greater than 100 μmol/L and often causes cardiovascular disease by the age of 30 years.15
A deficiency in the enzyme methylene tetrahydrofolate reductase (MTHFR) is a more common cause of mildly to moderately elevated plasma homocysteine levels.16 The MTHFR deficiency involves a variation at position 677 in the MTHFR gene in which cytosine is replaced by thymidine (thus called C677T or 677C>T).17 Ten percent of the population are homozygous for this variant (TT), 43% are heterozygous (CT), and 47% are unaffected (CC). Heterozygotes have slightly higher homocysteine levels than unaffected people, while people with the TT genotype have approximately 20% higher homocysteine levels.17
ELEVATED HOMOCYSTEINE IS COMMON
In a study of a population in Norway from 1992 to 1993, 8.5% had mild elevations in homocysteine (plasma levels 15–29.99 μmol/L), 0.8% had moderate elevations (30–99.99 μmol/L), and 0.02% had severe elevations (≥ 100 μmol/L).13,18 The prevalence of hyperhomocysteinemia in the United States is probably much lower, given that supplementation of white flour and cereal grains with folic acid has been mandatory since 1998, but this is not well described in the literature.
HOW GREAT IS THE RISK?
Studies over the past 10 to 20 years have shown that elevated homocysteine is a marker of risk of cardiovascular disease. The association was first noted in patients with cystathionine beta-synthase deficiency, who tend to have premature cardiovascular disease.
However, studies of patients with MTHFR 677C>T have yielded mixed results. Although several meta-analyses found up to a 42% higher rate of ischemic heart disease and stroke in patients homozygous for MTHFR 677C>T (the TT genotype) than in those with the CC genotype,17,19,20 two other large meta-analyses did not find an association between this variant and vascular risk.21,22
Nonetheless, in a meta-analysis of the association between homocysteine and cardiovascular disease, Wald et al17 found that for every 5-μmol/L increase in serum homocysteine concentration, the risk of ischemic heart disease increased 20% to 30%.
TRIALS OF HOMOCYSTEINE-LOWERING THERAPY HAVE HAD MIXED RESULTS
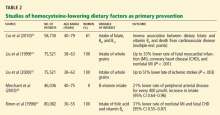
Primary prevention of cardiovascular disease
Given the finding that treatment with folic acid lowers homocysteine—initially noted in patients with homocystinuria—researchers hypothesized that treatment with folic acid, vitamin B6, and vitamin B12 would decrease the risk of cardiovascular disease.
Thus, in its recent evaluation of novel risk markers of cardiovascular disease, the United States Preventive Services Task Force 28,29 does not recommend measuring the plasma homocysteine level in the evaluation of either low-risk or intermediate-risk populations, finding no evidence that it adds any useful information in predicting major coronary events beyond what one could get from calculating the Framingham Risk Score. The task force also found no evidence that treating people who have elevated homocysteine levels decreases their risk of subsequent cardiovascular events.
In addition, a recent Cochrane Database review of eight randomized controlled trials in patients at low risk did not find a lower risk of myocardial infarction (fatal or nonfatal), stroke, or death from any cause in patients receiving B-complex vitamins.30
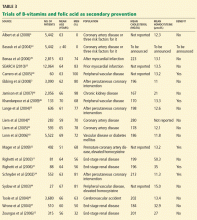
Secondary prevention of cardiovascular disease
Bazzano et al,47 in a meta-analysis published in 2006, evaluated 12 randomized controlled trials of folic acid supplementation in patients with known cardiovascular disease and did not find that treated patients had better cardiovascular outcomes. The mean homocysteine level was elevated (> 15 μmol/L) at baseline in only 4 of the 12 trials. However, in 1 of these 4 trials, there was no difference in outcomes comparing those with and without elevated homocysteine.31
Albert et al4 more recently evaluated the effect of a combination pill containing folic acid, vitamin B6, and vitamin B12 on cardiovascular events in women at high risk, ie, those with a history of cardiovascular disease or having three or more coronary risk factors. Treatment did not decrease the rate of the composite outcome of cardiovascular disease mortality, stroke, myocardial infarction, or coronary revascularization, although the homocysteine level decreased by a mean of 30% in the treated group. However, only 27.7% of the participants had an elevated homocysteine level. One might not expect patients to benefit from such treatment if they had normal homocysteine levels to begin with.
Ebbing et al,5 in a trial published in 2008, investigated the effect of folic acid, vitamin B12, and vitamin B6 supplements on the risks of death from any cause and of cardiovascular events in patients undergoing coronary angiography. Outcomes were no better in the treatment group than in the control group, despite a mean decrease in homocysteine level of 19%. However, over 90% of the participants had a normal homocysteine level.
Mager et al,32 in a study published in 2009, looked specifically at whether patients with coronary artery disease and elevated homocysteine levels (> 15 μmol/L) would benefit from folate-based vitamin therapy. In this subset, the incidence of death from any cause was lower in the treated group than in the control group (4% vs 32%, P < .001), an association that was not present in patients with normal homocysteine levels.
The SEARCH trial (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine),33 recently published, was a double-blind, randomized controlled trial of vitamin B12 and folic acid treatment in 12,064 patients who had survived a myocardial infarction. Although those who received the vitamin therapy had a 28% reduction in homocysteine level, no clinical benefit was demonstrated. Of note, 66% of the patients had a homocysteine level lower than 14 μmol/L at baseline.
Restenosis after angioplasty
Results are also mixed regarding whether folic acid supplements modify the risk of restenosis after coronary angioplasty.
Namazi et al48 evaluated the effect of folic acid supplementation on in-stent restenosis in 200 patients and found no difference between the treatment and placebo groups in the rates of either restenosis or target-vessel revascularization.
Schnyder et al49 evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on the rate of coronary restenosis (in cases of balloon angioplasty) or in-stent restenosis (if a stent was used). Patients receiving treatment had lower rates of restenosis or instent restenosis (40% vs 48%, P = .01) and of need for target-vessel revascularization (11% vs 22%, P = .047). The mean homocysteine level was not elevated in this study either, and the researchers did not analyze the outcomes according to whether patients had high or normal homocysteine levels.
Lange et al35 also evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on coronary in-stent restenosis. Paradoxically, the rate was higher with treatment in the overall group (mean homocysteine level 12.2 μmol/L), leading to a higher incidence of target-vessel revascularization. Patients who had a baseline elevation in homocysteine level had a nonsignificant trend toward a lower rate of in-stent restenosis.
Cerebrovascular and peripheral arterial disease
The evidence is also mixed for using folic acid and other B vitamins to prevent cerebrovascular disease and peripheral vascular disease. Although a 2007 meta-analysis found that folic acid supplementation decreased the risk of a first stroke by 18% (P = .045),50 a later meta-analysis contradicts this finding.51
A 2009 meta-analysis found that patients with peripheral arterial disease had higher homocysteine levels than controls, but it did not find any benefit from supplementation, owing to heterogeneity of the clinical end points used.52 Indeed, a 2009 Cochrane Database Systematic Review found that there were no adequate trials of the treatment of patients with peripheral vascular disease who have elevated plasma homocysteine.53
However, immediately after the Cochrane review was published, Khandanpour et al36 published the results of a trial of the effect of folic acid and 5-methyltetrahydrofolate (an active form of folic acid) supplementation on the ankle-brachial pressure index and the pulse-wave velocity in patients with peripheral arterial disease. These measures improved with 16 weeks of treatment. For the ankle-brachial pressure index, the P value was less than .01 for folic acid and .009 for 5-methyltetrahydrofolate; for the pulse-wave velocity, the P value was .051 for folic acid and .011 for 5-methyltetrahydrofolate.
Kidney disease
One could postulate that patients with end-stage renal disease or chronic kidney disease might benefit the most from folic acid supplementation, given the correlation of elevations in homocysteine levels with decline in glomerular filtration rate.
However, only one study found a lower rate of cardiovascular events with folic acid supplementation in dialysis patients, and the difference was not statistically significant (25% vs 36%, P < .08).31 Further, several studies found no benefit of folic acid supplementation in patients with chronic kidney disease.11,12,37
FUTURE DIRECTIONS AND RECOMMENDATIONS
Many experts have suggested that the existing evidence indicates that the homocysteine-lowering therapies folic acid, vitamin B6, and vitamin B12 do not lower the risk of cardiovascular disease.38,54–59 Indeed, the American Heart Association guidelines for cardiovascular disease prevention in women do not recommend folic acid supplementation to prevent cardiovascular disease.60 (Recommendations for men are the same as for women.) However, most of the clinical trials have not selected and treated patients with elevated homocysteine levels, but have instead included all patients regardless of homocysteine level.
At least two large ongoing trials are currently evaluating B-vitamin therapy for secondary prevention, but neither trial is looking specifically at patients with elevated homocysteine levels.61,62
Thus, instead of concluding that no patients could benefit from homocysteine-lowering treatment, future studies need to clarify:
- Whether patients with elevated homocysteine would benefit from such treatment
- At what level it would be appropriate to start treatment
- The appropriate target homocysteine level with treatment.
Particularly given the recent finding that folic acid supplementation may increase cancer risk,63 these questions need closer scrutiny.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc 2008; 83:1203–1212.
- Boers GH. The case for mild hyperhomocysteinaemia as a risk factor. J Inherit Metab Dis 1997; 20:301–306.
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 2004; 11(suppl 1):S56–S64.
- Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008; 299:2027–2036.
- Ebbing M, Bleie Ø, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008; 300:795–804.
- Butz LW, du Vigneaud V. The formation of a homologue of cystine by the decompensation of methionine with sulphuric acid. J Biol Chem 1932; 99:135–142.
- Gibson JB, Carson NA, Neill DW. Pathological findings in homocystinuria. J Clin Pathol 1964; 17:427–437.
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969; 56:111–128.
- Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 2009; 120:1893–1902.
- Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol 1999; 34:2002–2006.
- Righetti M, Ferrario GM, Milani S, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 2003; 9:PI19–PI24.
- Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol 2006; 47:1108–1116.
- Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge, UK: Cambridge University Press, 2001.
- Yap S, Boers GH, Wilcken B, et al. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol 2001; 21:2080–2085.
- McKusick V. 236200 Homocystinuria. In:McKusick V, editor. Mendelian Inheritance in Man. 10th ed. Baltimore, MD: The Johns Hopkins University Press, 1992:1444–1446.
- McKusick V. 236250 Homocystinuria due to deficiency of N(5,10)-methylenetetrahydrofolate reductase activity. In:McKusick V, editor. Mendelian Inheritance in Man. Baltimore, MD: The Johns Hopkins University Press, 1992:1447–1448.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325:1202.
- Nygård O, Vollset SE, Refsum H, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 1995; 274:1526–1533.
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG; MTHFR Studies Collaboration Group. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 2002; 288:2023–2031.
- Kelly PJ, Rosand J, Kistler JP, et al. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology 2002; 59:529–536.
- Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of MTHFR 677C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 2005; 331:1053.
- Brattström L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 1998; 98:2520–2526.
- Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A; Japan Collaborative Cohort Study Group. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan Collaborative Cohort Study. Stroke 2010; 41:1285–1289.
- Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr 1999; 70:412–419.
- Liu S, Manson JE, Stampfer MJ, et al. Whole grain consumption and risk of ischemic stroke in women: a prospective study. JAMA 2000; 284:1534–1540.
- Merchant AT, Hu FB, Spiegelman D, Willett WC, Rimm EB, Ascherio A. The use of B vitamin supplements and peripheral arterial disease risk in men are inversely related. J Nutr 2003; 133:2863–2867.
- Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998; 279:359–364.
- US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:474–482.
- Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the US Preventive Services Task Force. Ann Intern Med 2009; 151:496–507.
- Martí-Carvajal AJ, Solà I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2009; ( 4):CD006612.
- Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif 2006; 24:379–386.
- Mager A, Orvin K, Koren-Morag N, et al. Impact of homocysteine-lowering vitamin therapy on long-term outcome of patients with coronary artery disease. Am J Cardiol 2009; 104:745–749.
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group; Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010; 303:2486–2494.
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and percutaneous coronary intervention: the Swiss Heart Study: a randomized controlled trial. JAMA 2002; 288:973–979.
- Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004; 350:2673–2781.
- Khandanpour N, Armon MP, Jennings B, et al. Randomized clinical trial of folate supplementation in patients with peripheral arterial disease. Br J Surg 2009; 96:990–998.
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol 2004; 15:420–426.
- Loscalzo J. Homocysteine trials—clear outcomes for complex reasons. N Engl J Med 2006; 354:1629–1632.
- Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006; 354:1578–1588.
- Carrero JJ, López-Huertas E, Salmerón LM, Baró L, Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr 2005; 135:1393–1399.
- Liem AH, van Boven AJ, Veeger NJ, et al; Folic Acid on Risk Diminishment After Acute Myocarial Infarction Study Group. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol 2004; 93:175–179.
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005; 91:1213–1214.
- Lonn E, Yusuf S, Arnold MJ, et al; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006; 354:1567–1577.
- Sydow K, Schwedhelm E, Arakawa N, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res 2003; 57:244–252.
- Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004; 291:565–575.
- Jamison RL, Hartigan P, Kaufman JS, et al; Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA 2007; 2989:1163–1170. Erratum in JAMA 2008;300:170.
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006; 296:2720–2726.
- Namazi MH, Motamedi MR, Safi M, Vakili H, Saadat H, Nazari N. Efficacy of folic acid therapy for prevention of in-stent restenosis: a randomized clinical trial. Arch Iran Med 2006; 9:108–110.
- Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med 2001; 345:1593–1600.
- Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007; 369:1876–1882.
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic Acid in stroke prevention: a meta-analysis. Stroke 2010; 41:1205–1212.
- Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2009; 38:316–322.
- Hansrani M, Stansby G. Homocysteine lowering interventions for peripheral arterial disease and bypass grafts. Cochrane Database Syst Rev 2002; ( 3):CD003285.
- Mosca L. Novel cardiovascular risk factors: do they add value to your practice? Am Fam Physician 2003; 67:264,266.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Lonn E. Homocysteine-lowering B vitamin therapy in cardiovascular prevention—wrong again? JAMA 2008; 299:2086–2087.
- Milani RV, Lavie CJ. Homocysteine: the Rubik’s cube of cardiovascular risk factors. Mayo Clin Proc 2008; 83:1200–1202.
- Bazzano LA. Folic acid supplementation and cardiovascular disease: the state of the art. Am J Med Sci 2009; 338:48–49.
- Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: an update. Arch Cardiovasc Dis 2009; 102:847–854.
- Mosca L, Banka CL, Benjamin EJ; Expert Panel/Writing Group. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Bassuk SS, Albert CM, Cook NR, et al. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004; 13:99–117.
- SEARCH Study Collaborative Group; Bowman L, Armitage J, Bulbulia R, Parish S, Collins R. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am Heart J 2007; 154:815–823,823.e1–e6.
- Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009; 302:2119–2126.
Patients often ask primary care physicians and cardiologists about the measurement of biomarkers for cardiovascular disease and about the efficacy of preventive measures.
Although studies have shown that elevated homocysteine is a risk factor for cardiovascular and peripheral arterial disease1–3 and that supplementation with folic acid, vitamin B6, and vitamin B12 lowers homocysteine levels,4,5 it is unclear whether such supplementation prevents cardiovascular events. As a result, there is no consensus about whose homocysteine levels should be measured and who, if anyone, should receive homocysteine-lowering therapies.
The aim of this paper is to examine whether the evidence is sufficient to recommend homocysteine testing to guide the prevention and treatment of cardiovascular disease, or to recommend using folic acid, vitamin B6, and vitamin B12 for primary or secondary prevention of cardiovascular disease.
HISTORY OF HOMOCYSTEINE AS A RISK MARKER
Homocysteine is an amino acid formed from the metabolism of methionine, an essential amino acid derived from dietary protein. Although homocysteine was first isolated by Butz and du Vigneaud in 1932,6 it was not until 1964 that Gibson et al7 reported that patients with homocystinuria (more about this below) had vascular anomalies and arterial thrombosis. In 1969, McCully8 made the connection between elevated homocysteine levels and the risk of atherosclerosis.
Several possible mechanisms for the association between homocysteine and atherosclerosis have been demonstrated in experimental models. These include stimulation of smooth muscle growth, reduction in endothelial cell growth, impaired endothelial cell relaxation, decreased synthesis of high-density lipoprotein, promotion of autoimmune response, and accumulation of inflammatory monocytes in atherosclerotic plaques.3,9,10
In view of these findings, researchers have been evaluating whether homocysteine-lowering therapies decrease the risk of cardiovascular disease.
CAUSES OF ELEVATED PLASMA HOMOCYSTEINE
Vitamin deficiencies. Vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin), and folic acid are cofactors required for homocysteine metabolism, and deficiency in any or all of these leads to disruption of the relevant metabolic pathways.
Renal impairment. A low glomerular filtration rate has also been correlated with an elevated plasma homocysteine concentration. This makes sense, since the kidneys perform up to 70% of the clearance of homocysteine, although a cause-and-effect relationship is unclear.13
Inborn errors of homocysteine metabolism.Homocystinuria, ie, an abnormal elevation of homocysteine in the urine, is caused by several autosomal recessive disorders. People with these genetic variations have extremely high homocysteine levels.
A deficiency in the enzyme cystathionine beta-synthase is quite rare (the incidence in newborn babies has been found to be 1 in 344,000 worldwide and 1 in 65,000 in Ireland and Australia14), but leads to homocysteine levels greater than 100 μmol/L and often causes cardiovascular disease by the age of 30 years.15
A deficiency in the enzyme methylene tetrahydrofolate reductase (MTHFR) is a more common cause of mildly to moderately elevated plasma homocysteine levels.16 The MTHFR deficiency involves a variation at position 677 in the MTHFR gene in which cytosine is replaced by thymidine (thus called C677T or 677C>T).17 Ten percent of the population are homozygous for this variant (TT), 43% are heterozygous (CT), and 47% are unaffected (CC). Heterozygotes have slightly higher homocysteine levels than unaffected people, while people with the TT genotype have approximately 20% higher homocysteine levels.17
ELEVATED HOMOCYSTEINE IS COMMON
In a study of a population in Norway from 1992 to 1993, 8.5% had mild elevations in homocysteine (plasma levels 15–29.99 μmol/L), 0.8% had moderate elevations (30–99.99 μmol/L), and 0.02% had severe elevations (≥ 100 μmol/L).13,18 The prevalence of hyperhomocysteinemia in the United States is probably much lower, given that supplementation of white flour and cereal grains with folic acid has been mandatory since 1998, but this is not well described in the literature.
HOW GREAT IS THE RISK?
Studies over the past 10 to 20 years have shown that elevated homocysteine is a marker of risk of cardiovascular disease. The association was first noted in patients with cystathionine beta-synthase deficiency, who tend to have premature cardiovascular disease.
However, studies of patients with MTHFR 677C>T have yielded mixed results. Although several meta-analyses found up to a 42% higher rate of ischemic heart disease and stroke in patients homozygous for MTHFR 677C>T (the TT genotype) than in those with the CC genotype,17,19,20 two other large meta-analyses did not find an association between this variant and vascular risk.21,22
Nonetheless, in a meta-analysis of the association between homocysteine and cardiovascular disease, Wald et al17 found that for every 5-μmol/L increase in serum homocysteine concentration, the risk of ischemic heart disease increased 20% to 30%.
TRIALS OF HOMOCYSTEINE-LOWERING THERAPY HAVE HAD MIXED RESULTS
Primary prevention of cardiovascular disease
Given the finding that treatment with folic acid lowers homocysteine—initially noted in patients with homocystinuria—researchers hypothesized that treatment with folic acid, vitamin B6, and vitamin B12 would decrease the risk of cardiovascular disease.
Thus, in its recent evaluation of novel risk markers of cardiovascular disease, the United States Preventive Services Task Force 28,29 does not recommend measuring the plasma homocysteine level in the evaluation of either low-risk or intermediate-risk populations, finding no evidence that it adds any useful information in predicting major coronary events beyond what one could get from calculating the Framingham Risk Score. The task force also found no evidence that treating people who have elevated homocysteine levels decreases their risk of subsequent cardiovascular events.
In addition, a recent Cochrane Database review of eight randomized controlled trials in patients at low risk did not find a lower risk of myocardial infarction (fatal or nonfatal), stroke, or death from any cause in patients receiving B-complex vitamins.30
Secondary prevention of cardiovascular disease
Bazzano et al,47 in a meta-analysis published in 2006, evaluated 12 randomized controlled trials of folic acid supplementation in patients with known cardiovascular disease and did not find that treated patients had better cardiovascular outcomes. The mean homocysteine level was elevated (> 15 μmol/L) at baseline in only 4 of the 12 trials. However, in 1 of these 4 trials, there was no difference in outcomes comparing those with and without elevated homocysteine.31
Albert et al4 more recently evaluated the effect of a combination pill containing folic acid, vitamin B6, and vitamin B12 on cardiovascular events in women at high risk, ie, those with a history of cardiovascular disease or having three or more coronary risk factors. Treatment did not decrease the rate of the composite outcome of cardiovascular disease mortality, stroke, myocardial infarction, or coronary revascularization, although the homocysteine level decreased by a mean of 30% in the treated group. However, only 27.7% of the participants had an elevated homocysteine level. One might not expect patients to benefit from such treatment if they had normal homocysteine levels to begin with.
Ebbing et al,5 in a trial published in 2008, investigated the effect of folic acid, vitamin B12, and vitamin B6 supplements on the risks of death from any cause and of cardiovascular events in patients undergoing coronary angiography. Outcomes were no better in the treatment group than in the control group, despite a mean decrease in homocysteine level of 19%. However, over 90% of the participants had a normal homocysteine level.
Mager et al,32 in a study published in 2009, looked specifically at whether patients with coronary artery disease and elevated homocysteine levels (> 15 μmol/L) would benefit from folate-based vitamin therapy. In this subset, the incidence of death from any cause was lower in the treated group than in the control group (4% vs 32%, P < .001), an association that was not present in patients with normal homocysteine levels.
The SEARCH trial (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine),33 recently published, was a double-blind, randomized controlled trial of vitamin B12 and folic acid treatment in 12,064 patients who had survived a myocardial infarction. Although those who received the vitamin therapy had a 28% reduction in homocysteine level, no clinical benefit was demonstrated. Of note, 66% of the patients had a homocysteine level lower than 14 μmol/L at baseline.
Restenosis after angioplasty
Results are also mixed regarding whether folic acid supplements modify the risk of restenosis after coronary angioplasty.
Namazi et al48 evaluated the effect of folic acid supplementation on in-stent restenosis in 200 patients and found no difference between the treatment and placebo groups in the rates of either restenosis or target-vessel revascularization.
Schnyder et al49 evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on the rate of coronary restenosis (in cases of balloon angioplasty) or in-stent restenosis (if a stent was used). Patients receiving treatment had lower rates of restenosis or instent restenosis (40% vs 48%, P = .01) and of need for target-vessel revascularization (11% vs 22%, P = .047). The mean homocysteine level was not elevated in this study either, and the researchers did not analyze the outcomes according to whether patients had high or normal homocysteine levels.
Lange et al35 also evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on coronary in-stent restenosis. Paradoxically, the rate was higher with treatment in the overall group (mean homocysteine level 12.2 μmol/L), leading to a higher incidence of target-vessel revascularization. Patients who had a baseline elevation in homocysteine level had a nonsignificant trend toward a lower rate of in-stent restenosis.
Cerebrovascular and peripheral arterial disease
The evidence is also mixed for using folic acid and other B vitamins to prevent cerebrovascular disease and peripheral vascular disease. Although a 2007 meta-analysis found that folic acid supplementation decreased the risk of a first stroke by 18% (P = .045),50 a later meta-analysis contradicts this finding.51
A 2009 meta-analysis found that patients with peripheral arterial disease had higher homocysteine levels than controls, but it did not find any benefit from supplementation, owing to heterogeneity of the clinical end points used.52 Indeed, a 2009 Cochrane Database Systematic Review found that there were no adequate trials of the treatment of patients with peripheral vascular disease who have elevated plasma homocysteine.53
However, immediately after the Cochrane review was published, Khandanpour et al36 published the results of a trial of the effect of folic acid and 5-methyltetrahydrofolate (an active form of folic acid) supplementation on the ankle-brachial pressure index and the pulse-wave velocity in patients with peripheral arterial disease. These measures improved with 16 weeks of treatment. For the ankle-brachial pressure index, the P value was less than .01 for folic acid and .009 for 5-methyltetrahydrofolate; for the pulse-wave velocity, the P value was .051 for folic acid and .011 for 5-methyltetrahydrofolate.
Kidney disease
One could postulate that patients with end-stage renal disease or chronic kidney disease might benefit the most from folic acid supplementation, given the correlation of elevations in homocysteine levels with decline in glomerular filtration rate.
However, only one study found a lower rate of cardiovascular events with folic acid supplementation in dialysis patients, and the difference was not statistically significant (25% vs 36%, P < .08).31 Further, several studies found no benefit of folic acid supplementation in patients with chronic kidney disease.11,12,37
FUTURE DIRECTIONS AND RECOMMENDATIONS
Many experts have suggested that the existing evidence indicates that the homocysteine-lowering therapies folic acid, vitamin B6, and vitamin B12 do not lower the risk of cardiovascular disease.38,54–59 Indeed, the American Heart Association guidelines for cardiovascular disease prevention in women do not recommend folic acid supplementation to prevent cardiovascular disease.60 (Recommendations for men are the same as for women.) However, most of the clinical trials have not selected and treated patients with elevated homocysteine levels, but have instead included all patients regardless of homocysteine level.
At least two large ongoing trials are currently evaluating B-vitamin therapy for secondary prevention, but neither trial is looking specifically at patients with elevated homocysteine levels.61,62
Thus, instead of concluding that no patients could benefit from homocysteine-lowering treatment, future studies need to clarify:
- Whether patients with elevated homocysteine would benefit from such treatment
- At what level it would be appropriate to start treatment
- The appropriate target homocysteine level with treatment.
Particularly given the recent finding that folic acid supplementation may increase cancer risk,63 these questions need closer scrutiny.
Patients often ask primary care physicians and cardiologists about the measurement of biomarkers for cardiovascular disease and about the efficacy of preventive measures.
Although studies have shown that elevated homocysteine is a risk factor for cardiovascular and peripheral arterial disease1–3 and that supplementation with folic acid, vitamin B6, and vitamin B12 lowers homocysteine levels,4,5 it is unclear whether such supplementation prevents cardiovascular events. As a result, there is no consensus about whose homocysteine levels should be measured and who, if anyone, should receive homocysteine-lowering therapies.
The aim of this paper is to examine whether the evidence is sufficient to recommend homocysteine testing to guide the prevention and treatment of cardiovascular disease, or to recommend using folic acid, vitamin B6, and vitamin B12 for primary or secondary prevention of cardiovascular disease.
HISTORY OF HOMOCYSTEINE AS A RISK MARKER
Homocysteine is an amino acid formed from the metabolism of methionine, an essential amino acid derived from dietary protein. Although homocysteine was first isolated by Butz and du Vigneaud in 1932,6 it was not until 1964 that Gibson et al7 reported that patients with homocystinuria (more about this below) had vascular anomalies and arterial thrombosis. In 1969, McCully8 made the connection between elevated homocysteine levels and the risk of atherosclerosis.
Several possible mechanisms for the association between homocysteine and atherosclerosis have been demonstrated in experimental models. These include stimulation of smooth muscle growth, reduction in endothelial cell growth, impaired endothelial cell relaxation, decreased synthesis of high-density lipoprotein, promotion of autoimmune response, and accumulation of inflammatory monocytes in atherosclerotic plaques.3,9,10
In view of these findings, researchers have been evaluating whether homocysteine-lowering therapies decrease the risk of cardiovascular disease.
CAUSES OF ELEVATED PLASMA HOMOCYSTEINE
Vitamin deficiencies. Vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin), and folic acid are cofactors required for homocysteine metabolism, and deficiency in any or all of these leads to disruption of the relevant metabolic pathways.
Renal impairment. A low glomerular filtration rate has also been correlated with an elevated plasma homocysteine concentration. This makes sense, since the kidneys perform up to 70% of the clearance of homocysteine, although a cause-and-effect relationship is unclear.13
Inborn errors of homocysteine metabolism.Homocystinuria, ie, an abnormal elevation of homocysteine in the urine, is caused by several autosomal recessive disorders. People with these genetic variations have extremely high homocysteine levels.
A deficiency in the enzyme cystathionine beta-synthase is quite rare (the incidence in newborn babies has been found to be 1 in 344,000 worldwide and 1 in 65,000 in Ireland and Australia14), but leads to homocysteine levels greater than 100 μmol/L and often causes cardiovascular disease by the age of 30 years.15
A deficiency in the enzyme methylene tetrahydrofolate reductase (MTHFR) is a more common cause of mildly to moderately elevated plasma homocysteine levels.16 The MTHFR deficiency involves a variation at position 677 in the MTHFR gene in which cytosine is replaced by thymidine (thus called C677T or 677C>T).17 Ten percent of the population are homozygous for this variant (TT), 43% are heterozygous (CT), and 47% are unaffected (CC). Heterozygotes have slightly higher homocysteine levels than unaffected people, while people with the TT genotype have approximately 20% higher homocysteine levels.17
ELEVATED HOMOCYSTEINE IS COMMON
In a study of a population in Norway from 1992 to 1993, 8.5% had mild elevations in homocysteine (plasma levels 15–29.99 μmol/L), 0.8% had moderate elevations (30–99.99 μmol/L), and 0.02% had severe elevations (≥ 100 μmol/L).13,18 The prevalence of hyperhomocysteinemia in the United States is probably much lower, given that supplementation of white flour and cereal grains with folic acid has been mandatory since 1998, but this is not well described in the literature.
HOW GREAT IS THE RISK?
Studies over the past 10 to 20 years have shown that elevated homocysteine is a marker of risk of cardiovascular disease. The association was first noted in patients with cystathionine beta-synthase deficiency, who tend to have premature cardiovascular disease.
However, studies of patients with MTHFR 677C>T have yielded mixed results. Although several meta-analyses found up to a 42% higher rate of ischemic heart disease and stroke in patients homozygous for MTHFR 677C>T (the TT genotype) than in those with the CC genotype,17,19,20 two other large meta-analyses did not find an association between this variant and vascular risk.21,22
Nonetheless, in a meta-analysis of the association between homocysteine and cardiovascular disease, Wald et al17 found that for every 5-μmol/L increase in serum homocysteine concentration, the risk of ischemic heart disease increased 20% to 30%.
TRIALS OF HOMOCYSTEINE-LOWERING THERAPY HAVE HAD MIXED RESULTS
Primary prevention of cardiovascular disease
Given the finding that treatment with folic acid lowers homocysteine—initially noted in patients with homocystinuria—researchers hypothesized that treatment with folic acid, vitamin B6, and vitamin B12 would decrease the risk of cardiovascular disease.
Thus, in its recent evaluation of novel risk markers of cardiovascular disease, the United States Preventive Services Task Force 28,29 does not recommend measuring the plasma homocysteine level in the evaluation of either low-risk or intermediate-risk populations, finding no evidence that it adds any useful information in predicting major coronary events beyond what one could get from calculating the Framingham Risk Score. The task force also found no evidence that treating people who have elevated homocysteine levels decreases their risk of subsequent cardiovascular events.
In addition, a recent Cochrane Database review of eight randomized controlled trials in patients at low risk did not find a lower risk of myocardial infarction (fatal or nonfatal), stroke, or death from any cause in patients receiving B-complex vitamins.30
Secondary prevention of cardiovascular disease
Bazzano et al,47 in a meta-analysis published in 2006, evaluated 12 randomized controlled trials of folic acid supplementation in patients with known cardiovascular disease and did not find that treated patients had better cardiovascular outcomes. The mean homocysteine level was elevated (> 15 μmol/L) at baseline in only 4 of the 12 trials. However, in 1 of these 4 trials, there was no difference in outcomes comparing those with and without elevated homocysteine.31
Albert et al4 more recently evaluated the effect of a combination pill containing folic acid, vitamin B6, and vitamin B12 on cardiovascular events in women at high risk, ie, those with a history of cardiovascular disease or having three or more coronary risk factors. Treatment did not decrease the rate of the composite outcome of cardiovascular disease mortality, stroke, myocardial infarction, or coronary revascularization, although the homocysteine level decreased by a mean of 30% in the treated group. However, only 27.7% of the participants had an elevated homocysteine level. One might not expect patients to benefit from such treatment if they had normal homocysteine levels to begin with.
Ebbing et al,5 in a trial published in 2008, investigated the effect of folic acid, vitamin B12, and vitamin B6 supplements on the risks of death from any cause and of cardiovascular events in patients undergoing coronary angiography. Outcomes were no better in the treatment group than in the control group, despite a mean decrease in homocysteine level of 19%. However, over 90% of the participants had a normal homocysteine level.
Mager et al,32 in a study published in 2009, looked specifically at whether patients with coronary artery disease and elevated homocysteine levels (> 15 μmol/L) would benefit from folate-based vitamin therapy. In this subset, the incidence of death from any cause was lower in the treated group than in the control group (4% vs 32%, P < .001), an association that was not present in patients with normal homocysteine levels.
The SEARCH trial (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine),33 recently published, was a double-blind, randomized controlled trial of vitamin B12 and folic acid treatment in 12,064 patients who had survived a myocardial infarction. Although those who received the vitamin therapy had a 28% reduction in homocysteine level, no clinical benefit was demonstrated. Of note, 66% of the patients had a homocysteine level lower than 14 μmol/L at baseline.
Restenosis after angioplasty
Results are also mixed regarding whether folic acid supplements modify the risk of restenosis after coronary angioplasty.
Namazi et al48 evaluated the effect of folic acid supplementation on in-stent restenosis in 200 patients and found no difference between the treatment and placebo groups in the rates of either restenosis or target-vessel revascularization.
Schnyder et al49 evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on the rate of coronary restenosis (in cases of balloon angioplasty) or in-stent restenosis (if a stent was used). Patients receiving treatment had lower rates of restenosis or instent restenosis (40% vs 48%, P = .01) and of need for target-vessel revascularization (11% vs 22%, P = .047). The mean homocysteine level was not elevated in this study either, and the researchers did not analyze the outcomes according to whether patients had high or normal homocysteine levels.
Lange et al35 also evaluated the effect of folic acid, vitamin B6, and vitamin B12 treatment on coronary in-stent restenosis. Paradoxically, the rate was higher with treatment in the overall group (mean homocysteine level 12.2 μmol/L), leading to a higher incidence of target-vessel revascularization. Patients who had a baseline elevation in homocysteine level had a nonsignificant trend toward a lower rate of in-stent restenosis.
Cerebrovascular and peripheral arterial disease
The evidence is also mixed for using folic acid and other B vitamins to prevent cerebrovascular disease and peripheral vascular disease. Although a 2007 meta-analysis found that folic acid supplementation decreased the risk of a first stroke by 18% (P = .045),50 a later meta-analysis contradicts this finding.51
A 2009 meta-analysis found that patients with peripheral arterial disease had higher homocysteine levels than controls, but it did not find any benefit from supplementation, owing to heterogeneity of the clinical end points used.52 Indeed, a 2009 Cochrane Database Systematic Review found that there were no adequate trials of the treatment of patients with peripheral vascular disease who have elevated plasma homocysteine.53
However, immediately after the Cochrane review was published, Khandanpour et al36 published the results of a trial of the effect of folic acid and 5-methyltetrahydrofolate (an active form of folic acid) supplementation on the ankle-brachial pressure index and the pulse-wave velocity in patients with peripheral arterial disease. These measures improved with 16 weeks of treatment. For the ankle-brachial pressure index, the P value was less than .01 for folic acid and .009 for 5-methyltetrahydrofolate; for the pulse-wave velocity, the P value was .051 for folic acid and .011 for 5-methyltetrahydrofolate.
Kidney disease
One could postulate that patients with end-stage renal disease or chronic kidney disease might benefit the most from folic acid supplementation, given the correlation of elevations in homocysteine levels with decline in glomerular filtration rate.
However, only one study found a lower rate of cardiovascular events with folic acid supplementation in dialysis patients, and the difference was not statistically significant (25% vs 36%, P < .08).31 Further, several studies found no benefit of folic acid supplementation in patients with chronic kidney disease.11,12,37
FUTURE DIRECTIONS AND RECOMMENDATIONS
Many experts have suggested that the existing evidence indicates that the homocysteine-lowering therapies folic acid, vitamin B6, and vitamin B12 do not lower the risk of cardiovascular disease.38,54–59 Indeed, the American Heart Association guidelines for cardiovascular disease prevention in women do not recommend folic acid supplementation to prevent cardiovascular disease.60 (Recommendations for men are the same as for women.) However, most of the clinical trials have not selected and treated patients with elevated homocysteine levels, but have instead included all patients regardless of homocysteine level.
At least two large ongoing trials are currently evaluating B-vitamin therapy for secondary prevention, but neither trial is looking specifically at patients with elevated homocysteine levels.61,62
Thus, instead of concluding that no patients could benefit from homocysteine-lowering treatment, future studies need to clarify:
- Whether patients with elevated homocysteine would benefit from such treatment
- At what level it would be appropriate to start treatment
- The appropriate target homocysteine level with treatment.
Particularly given the recent finding that folic acid supplementation may increase cancer risk,63 these questions need closer scrutiny.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc 2008; 83:1203–1212.
- Boers GH. The case for mild hyperhomocysteinaemia as a risk factor. J Inherit Metab Dis 1997; 20:301–306.
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 2004; 11(suppl 1):S56–S64.
- Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008; 299:2027–2036.
- Ebbing M, Bleie Ø, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008; 300:795–804.
- Butz LW, du Vigneaud V. The formation of a homologue of cystine by the decompensation of methionine with sulphuric acid. J Biol Chem 1932; 99:135–142.
- Gibson JB, Carson NA, Neill DW. Pathological findings in homocystinuria. J Clin Pathol 1964; 17:427–437.
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969; 56:111–128.
- Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 2009; 120:1893–1902.
- Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol 1999; 34:2002–2006.
- Righetti M, Ferrario GM, Milani S, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 2003; 9:PI19–PI24.
- Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol 2006; 47:1108–1116.
- Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge, UK: Cambridge University Press, 2001.
- Yap S, Boers GH, Wilcken B, et al. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol 2001; 21:2080–2085.
- McKusick V. 236200 Homocystinuria. In:McKusick V, editor. Mendelian Inheritance in Man. 10th ed. Baltimore, MD: The Johns Hopkins University Press, 1992:1444–1446.
- McKusick V. 236250 Homocystinuria due to deficiency of N(5,10)-methylenetetrahydrofolate reductase activity. In:McKusick V, editor. Mendelian Inheritance in Man. Baltimore, MD: The Johns Hopkins University Press, 1992:1447–1448.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325:1202.
- Nygård O, Vollset SE, Refsum H, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 1995; 274:1526–1533.
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG; MTHFR Studies Collaboration Group. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 2002; 288:2023–2031.
- Kelly PJ, Rosand J, Kistler JP, et al. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology 2002; 59:529–536.
- Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of MTHFR 677C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 2005; 331:1053.
- Brattström L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 1998; 98:2520–2526.
- Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A; Japan Collaborative Cohort Study Group. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan Collaborative Cohort Study. Stroke 2010; 41:1285–1289.
- Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr 1999; 70:412–419.
- Liu S, Manson JE, Stampfer MJ, et al. Whole grain consumption and risk of ischemic stroke in women: a prospective study. JAMA 2000; 284:1534–1540.
- Merchant AT, Hu FB, Spiegelman D, Willett WC, Rimm EB, Ascherio A. The use of B vitamin supplements and peripheral arterial disease risk in men are inversely related. J Nutr 2003; 133:2863–2867.
- Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998; 279:359–364.
- US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:474–482.
- Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the US Preventive Services Task Force. Ann Intern Med 2009; 151:496–507.
- Martí-Carvajal AJ, Solà I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2009; ( 4):CD006612.
- Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif 2006; 24:379–386.
- Mager A, Orvin K, Koren-Morag N, et al. Impact of homocysteine-lowering vitamin therapy on long-term outcome of patients with coronary artery disease. Am J Cardiol 2009; 104:745–749.
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group; Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010; 303:2486–2494.
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and percutaneous coronary intervention: the Swiss Heart Study: a randomized controlled trial. JAMA 2002; 288:973–979.
- Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004; 350:2673–2781.
- Khandanpour N, Armon MP, Jennings B, et al. Randomized clinical trial of folate supplementation in patients with peripheral arterial disease. Br J Surg 2009; 96:990–998.
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol 2004; 15:420–426.
- Loscalzo J. Homocysteine trials—clear outcomes for complex reasons. N Engl J Med 2006; 354:1629–1632.
- Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006; 354:1578–1588.
- Carrero JJ, López-Huertas E, Salmerón LM, Baró L, Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr 2005; 135:1393–1399.
- Liem AH, van Boven AJ, Veeger NJ, et al; Folic Acid on Risk Diminishment After Acute Myocarial Infarction Study Group. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol 2004; 93:175–179.
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005; 91:1213–1214.
- Lonn E, Yusuf S, Arnold MJ, et al; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006; 354:1567–1577.
- Sydow K, Schwedhelm E, Arakawa N, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res 2003; 57:244–252.
- Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004; 291:565–575.
- Jamison RL, Hartigan P, Kaufman JS, et al; Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA 2007; 2989:1163–1170. Erratum in JAMA 2008;300:170.
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006; 296:2720–2726.
- Namazi MH, Motamedi MR, Safi M, Vakili H, Saadat H, Nazari N. Efficacy of folic acid therapy for prevention of in-stent restenosis: a randomized clinical trial. Arch Iran Med 2006; 9:108–110.
- Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med 2001; 345:1593–1600.
- Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007; 369:1876–1882.
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic Acid in stroke prevention: a meta-analysis. Stroke 2010; 41:1205–1212.
- Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2009; 38:316–322.
- Hansrani M, Stansby G. Homocysteine lowering interventions for peripheral arterial disease and bypass grafts. Cochrane Database Syst Rev 2002; ( 3):CD003285.
- Mosca L. Novel cardiovascular risk factors: do they add value to your practice? Am Fam Physician 2003; 67:264,266.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Lonn E. Homocysteine-lowering B vitamin therapy in cardiovascular prevention—wrong again? JAMA 2008; 299:2086–2087.
- Milani RV, Lavie CJ. Homocysteine: the Rubik’s cube of cardiovascular risk factors. Mayo Clin Proc 2008; 83:1200–1202.
- Bazzano LA. Folic acid supplementation and cardiovascular disease: the state of the art. Am J Med Sci 2009; 338:48–49.
- Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: an update. Arch Cardiovasc Dis 2009; 102:847–854.
- Mosca L, Banka CL, Benjamin EJ; Expert Panel/Writing Group. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Bassuk SS, Albert CM, Cook NR, et al. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004; 13:99–117.
- SEARCH Study Collaborative Group; Bowman L, Armitage J, Bulbulia R, Parish S, Collins R. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am Heart J 2007; 154:815–823,823.e1–e6.
- Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009; 302:2119–2126.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc 2008; 83:1203–1212.
- Boers GH. The case for mild hyperhomocysteinaemia as a risk factor. J Inherit Metab Dis 1997; 20:301–306.
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 2004; 11(suppl 1):S56–S64.
- Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008; 299:2027–2036.
- Ebbing M, Bleie Ø, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008; 300:795–804.
- Butz LW, du Vigneaud V. The formation of a homologue of cystine by the decompensation of methionine with sulphuric acid. J Biol Chem 1932; 99:135–142.
- Gibson JB, Carson NA, Neill DW. Pathological findings in homocystinuria. J Clin Pathol 1964; 17:427–437.
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969; 56:111–128.
- Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 2009; 120:1893–1902.
- Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol 1999; 34:2002–2006.
- Righetti M, Ferrario GM, Milani S, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 2003; 9:PI19–PI24.
- Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol 2006; 47:1108–1116.
- Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge, UK: Cambridge University Press, 2001.
- Yap S, Boers GH, Wilcken B, et al. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol 2001; 21:2080–2085.
- McKusick V. 236200 Homocystinuria. In:McKusick V, editor. Mendelian Inheritance in Man. 10th ed. Baltimore, MD: The Johns Hopkins University Press, 1992:1444–1446.
- McKusick V. 236250 Homocystinuria due to deficiency of N(5,10)-methylenetetrahydrofolate reductase activity. In:McKusick V, editor. Mendelian Inheritance in Man. Baltimore, MD: The Johns Hopkins University Press, 1992:1447–1448.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325:1202.
- Nygård O, Vollset SE, Refsum H, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 1995; 274:1526–1533.
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG; MTHFR Studies Collaboration Group. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 2002; 288:2023–2031.
- Kelly PJ, Rosand J, Kistler JP, et al. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology 2002; 59:529–536.
- Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of MTHFR 677C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 2005; 331:1053.
- Brattström L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 1998; 98:2520–2526.
- Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A; Japan Collaborative Cohort Study Group. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan Collaborative Cohort Study. Stroke 2010; 41:1285–1289.
- Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr 1999; 70:412–419.
- Liu S, Manson JE, Stampfer MJ, et al. Whole grain consumption and risk of ischemic stroke in women: a prospective study. JAMA 2000; 284:1534–1540.
- Merchant AT, Hu FB, Spiegelman D, Willett WC, Rimm EB, Ascherio A. The use of B vitamin supplements and peripheral arterial disease risk in men are inversely related. J Nutr 2003; 133:2863–2867.
- Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998; 279:359–364.
- US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:474–482.
- Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the US Preventive Services Task Force. Ann Intern Med 2009; 151:496–507.
- Martí-Carvajal AJ, Solà I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2009; ( 4):CD006612.
- Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif 2006; 24:379–386.
- Mager A, Orvin K, Koren-Morag N, et al. Impact of homocysteine-lowering vitamin therapy on long-term outcome of patients with coronary artery disease. Am J Cardiol 2009; 104:745–749.
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group; Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010; 303:2486–2494.
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and percutaneous coronary intervention: the Swiss Heart Study: a randomized controlled trial. JAMA 2002; 288:973–979.
- Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004; 350:2673–2781.
- Khandanpour N, Armon MP, Jennings B, et al. Randomized clinical trial of folate supplementation in patients with peripheral arterial disease. Br J Surg 2009; 96:990–998.
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol 2004; 15:420–426.
- Loscalzo J. Homocysteine trials—clear outcomes for complex reasons. N Engl J Med 2006; 354:1629–1632.
- Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006; 354:1578–1588.
- Carrero JJ, López-Huertas E, Salmerón LM, Baró L, Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr 2005; 135:1393–1399.
- Liem AH, van Boven AJ, Veeger NJ, et al; Folic Acid on Risk Diminishment After Acute Myocarial Infarction Study Group. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. Int J Cardiol 2004; 93:175–179.
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005; 91:1213–1214.
- Lonn E, Yusuf S, Arnold MJ, et al; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006; 354:1567–1577.
- Sydow K, Schwedhelm E, Arakawa N, et al. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res 2003; 57:244–252.
- Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004; 291:565–575.
- Jamison RL, Hartigan P, Kaufman JS, et al; Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA 2007; 2989:1163–1170. Erratum in JAMA 2008;300:170.
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006; 296:2720–2726.
- Namazi MH, Motamedi MR, Safi M, Vakili H, Saadat H, Nazari N. Efficacy of folic acid therapy for prevention of in-stent restenosis: a randomized clinical trial. Arch Iran Med 2006; 9:108–110.
- Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med 2001; 345:1593–1600.
- Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007; 369:1876–1882.
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic Acid in stroke prevention: a meta-analysis. Stroke 2010; 41:1205–1212.
- Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2009; 38:316–322.
- Hansrani M, Stansby G. Homocysteine lowering interventions for peripheral arterial disease and bypass grafts. Cochrane Database Syst Rev 2002; ( 3):CD003285.
- Mosca L. Novel cardiovascular risk factors: do they add value to your practice? Am Fam Physician 2003; 67:264,266.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Lonn E. Homocysteine-lowering B vitamin therapy in cardiovascular prevention—wrong again? JAMA 2008; 299:2086–2087.
- Milani RV, Lavie CJ. Homocysteine: the Rubik’s cube of cardiovascular risk factors. Mayo Clin Proc 2008; 83:1200–1202.
- Bazzano LA. Folic acid supplementation and cardiovascular disease: the state of the art. Am J Med Sci 2009; 338:48–49.
- Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: an update. Arch Cardiovasc Dis 2009; 102:847–854.
- Mosca L, Banka CL, Benjamin EJ; Expert Panel/Writing Group. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007; 115:1481–1501.
- Bassuk SS, Albert CM, Cook NR, et al. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004; 13:99–117.
- SEARCH Study Collaborative Group; Bowman L, Armitage J, Bulbulia R, Parish S, Collins R. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am Heart J 2007; 154:815–823,823.e1–e6.
- Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009; 302:2119–2126.
KEY POINTS
- Factors that can cause the plasma homocysteine concentration to be high include deficiencies of vitamin B6, vitamin B12, and folic acid; renal insufficiency; and genetic variants in enzymes responsible for homocysteine metabolism.
- Higher plasma homocysteine levels are associated with a higher risk of cardiovascular, cerebrovascular, and peripheral arterial disease.
- Supplementation of B vitamins and folic acid can lower plasma homocysteine levels.
- Randomized controlled trials of supplementation to prevent cardiovascular events and other adverse outcomes have had mostly negative results. However, most patients in these trials had normal baseline plasma homocysteine levels.
- Needed are randomized trials to see if supplementation improves outcomes in patients with high homocysteine levels.