User login
Hepatitis b virus (HBV) infection is sometimes challenging to manage because the disease has several stages and many clinical scenarios. HBV-infected patients are a very heterogeneous group, and we cannot apply a single management approach to all.
An understanding of the natural history of HBV infection and its diagnosis, which we reviewed in last month’s issue of this Journal1, is critical to understanding how to manage HBV infection.
In this article, we will review the principles of HBV management in adults, including those on immunosuppressant therapy and pregnant women, and guidelines for HBV vaccination.
WORKUP FOR HBV INFECTION
Once the diagnosis of HBV infection is made,1 a full management strategy should be formulated, as outlined below.
History
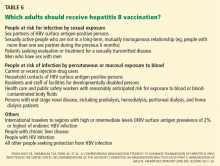
When and how did the patient acquire HBV? This information is important to know when making treatment decisions. For example, most acute, adult-onset cases (eg, acquired recently via sexual contact or parenteral drug abuse) resolve spontaneously within a few months, whereas most chronic cases (defined as being positive for HBV surface antigen for more than 6 months) were acquired at birth or in early childhood. Therefore, we should try to determine if the patient’s mother, siblings, household contacts, and sexual partners are positive for HBV surface antigen or have risk factors for HBV infection1; those without infection or immunity to HBV should be vaccinated.
People at risk of HBV infection include:
- Parenteral drug users
- People with multiple sexual partners
- Household contacts and sexual partners of people positive for HBV surface antigen
- Infants born to HBV-infected mothers
- Patients and staff in custodial institutions for the developmentally disabled
- Recipients of certain plasma-derived products (including patients with congenital coagulation defects)
- Hemodialysis patients
- Health and public-safety workers who have contact with blood
- People born in areas where HBV is endemic, and their children.1
Does the patient have risk factors for other infections? Especially look for risk factors for human immunodeficiency virus (HIV) infection (eg, intravenous drug users and men having sex with men) and hepatitis D virus (intravenous drug users and patients from countries where hepatitis D virus infection is common, particularly Eastern Europe, Mediterranean countries, and the Amazon basin).
Does the patient have other modifiable risk factors for progressive liver disease, particularly alcohol abuse and obesity?
Does the patient have symptoms or signs of cirrhosis or hepatocellular carcinoma? Symptoms and signs that involve multiple systems could be extrahepatic manifestations of HBV infection, such as polyarteritis nodosa, which causes abdominal pain, arthralgia, hypertension, and asymmetric polyneuropathy.
Baseline laboratory evaluation
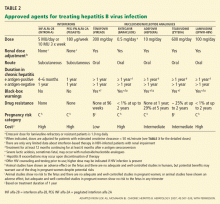
At baseline we should obtain a complete blood count, blood urea nitrogen level, serum creatinine level, liver profile, prothrombin time, urinalysis, and HBV serologic markers. In addition, HBV DNA can be detected in the serum at levels as low as 60 IU/mL, and it should be measured in the initial evaluation to establish a baseline before starting antiviral therapy in patients with chronic HBV infection and subsequently to monitor the response.
All patients with chronic HBV infection should also be tested for serologic markers of hepatitis A and hepatitis C; patients at risk of HIV and hepatitis D should also be tested for these diseases.
Not all patients need liver biopsy
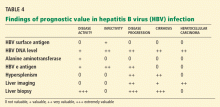
Liver biopsy is the most accurate tool for staging the degree of HBV-related hepatic fibrosis in patients who have no obvious clinical manifestations of cirrhosis.
Not all patients with HBV infection need a biopsy, however. In patients with acute HBV infection, liver biopsy has no benefit except if concomitant pathology (eg, iron overload, nonalcoholic steatohepatitis, or alcoholic steatohepatitis) is suspected. In patients with chronic hepatitis B, liver biopsy is helpful when the viral load alone does not provide sufficient guidance for treatment, eg, when the viral load is less than 2 × 104 IU/mL in a patient positive for hepatitis e antigen or less than 2 × 103 IU/mL in a patient negative for hepatitis e antigen. (The presence of e antigen is a marker of HBV replication and infectivity.1) Biopsy should also be considered in those who have been infected a long time (eg, more than 10 years), because they may have occult cirrhosis, and if they do they may need to undergo antiviral treatment, endoscopy to look for varices, and surveillance for liver cancer.
In some situations it is easy to decide whether antiviral therapy is indicated without resorting to liver biopsy.
We would treat:
- A patient positive for HBV e antigen for more than 6 months, whose HBV DNA level is higher than 2 × 104 IU/mL and whose alanine aminotransferase (ALT) level is high
- A patient with HBV for more than 6 months who is negative for e antigen and who has an HBV DNA level higher than 2 × 103 IU/mL and elevated ALT
- A patient with compensated HBV cirrhosis and an HBV DNA level higher than 2 × 103 IU/mL
- A patient with HBV cirrhosis with decompensation and any detectable HBV DNA.
We would not treat:
- An HBV carrier with a normal ALT level and an HBV DNA level that is lower than 2 × 104 IU/mL or undetectable.
If a patient does not fit into one of these categories but has HBV DNA, a liver biopsy showing significant necroinflammation or fibrosis would be an indication for treatment.
ANTIVIRAL THERAPY
Below, we summarize the main principles of anti-HBV therapy, emphasizing whether to treat and with which agent. Treatment of HBV infection in patients who are also infected with HIV or hepatitis C virus and in those with resistant or refractory hepatitis B is not within the scope of this article.
Acute infection rarely needs treatment
Acute, adult-acquired HBV infection is self-limited in most cases,1 and antiviral therapy is not routinely indicated.
In the rare cases of acute liver failure related to acute HBV infection, use of a nucleoside or nucleotide analogue reverse transcriptase inhibitor (nucleoside/nucleotide analogues) has been recommended, although no properly designed studies have been done.2,3 This recommendation is based on anecdotal experience, the relative safety of the antiviral agents, the serious nature of acute liver failure, and the possible need for emergency liver transplantation that requires prophylaxis against recurrence.
The nucleoside/nucleotide analogues that have been recommended in acute liver failure are lamivudine (Epivir), telbivudine (Tyzeka), and entecavir (Baraclude)—but not adefovir (Hepsera), which has a slow action and potential nephrotoxicity. Interferon drugs are contraindicated because they frequently cause side effects and can worsen hepatitis.4
Patients with acute liver failure should be referred promptly to a liver transplant center, and other management measures should also be started in a timely fashion.
In chronic HBV infection, treatment decisions are individualized
In chronic hepatitis B (ie, lasting > 6 months), treatment decisions should be based on the patient’s clinical situation and test results. The route and duration of infection (if known), history of previous hepatitis flares, ALT levels, current and previous HBV serologic test results and DNA levels, findings on liver biopsy (if previously done), and clinically suspected cirrhosis are all important to consider when deciding whether antiviral therapy is needed.
For many patients with chronic HBV infection, observation without antiviral therapy is warranted, eg:
- Young patients (< 30 years old) who acquired HBV at birth and who have persistently normal ALT levels with no evidence of advanced liver disease, regardless of their HBV DNA level (immune tolerance phase)
- Chronic inactive carriers who have no e antigen, persistently normal ALT levels, and very low or undetectable levels of HBV DNA without evidence of significant liver injury.
These patients can be managed by internists by close monitoring for hepatitis flares with serial ALT measurements along with other general management measures.
Antiviral agents for chronic HBV infection
An ideal agent for treating hepatitis B does not exist. Trade-offs are the essence of agent selection.
Interferons, the first drugs shown to be effective against HBV, can in some respects be considered the best available initial choice, especially in patients positive for hepatitis e antigen. Interferons have numerous side effects but, unlike all the other options, they have a well-defined duration of treatment (4–6 months in patients positive for e antigen). The principal goal of this therapy is disappearance of e antigen.
Interferon-based therapy is not recommended in patients with cirrhosis, however, because of the risk of hepatic decompensation associated with interferon-related flares of hepatitis.4
Although single-agent antiviral therapy may someday be replaced by a multidrug regimen, the data so far are not sufficiently robust to recommend multidrug regimens except possibly in cases of established drug resistance.
Adjunctive management
Vaccinations. All patients with chronic hepatitis B should be vaccinated against hepatitis A if serologic testing indicates they have no immunity to it. Influenza and pneumococcal vaccines are recommended for all patients with chronic liver disease.6
Alcohol rehabilitation. Patients who abuse alcohol should be counseled, and many need consultation with a psychosocial care provider for alcohol rehabilitation.
Smoking cessation. Cigarette smoking is linked to a higher risk of hepatocellular carcinoma in patients with chronic liver disease, including chronic HBV infection.7 Therefore, smokers should be counseled to quit.
Surveillance for hepatocellular carcinoma. Hepatocellular carcinoma can occur in patients with chronic hepatitis B, in most cases on top of cirrhosis, although important exceptions exist. The American Association for the Study of Liver Diseases recommends surveillance for hepatocellular carcinoma in all HBV carriers with cirrhosis and in the following groups regardless of whether they have cirrhosis8:
- Asian men age 40 and older
- Asian women age 50 and older
- African patients age 20 and older
- Patients with a family history of hepatocellular carcinoma
- Possibly, those with high HBV DNA levels and ongoing inflammatory activity.
In the United States, liver ultrasonography and alpha fetoprotein measurement every 6 to 12 months is a reasonable strategy.
If there is evidence of cirrhosis, esophagogastroduodenoscopy is recommended to screen for esophageal and gastric varices.
SCREEN BEFORE CHEMOTHERAPY OR IMMUNOSUPPRESSIVE THERAPY
When patients who are positive for HBV surface antigen undergo immunosuppressive therapy or cancer chemotherapy, from 20% to 50% develop reactivated HBV infection with high HBV viral loads. Even patients who have resolved hepatitis B (ie, negative for HBV surface antigen and positive for surface antibody) may experience hepatitis B reactivation, with serious consequences. Hepatic decompensation and death have been reported during and after chemotherapy, especially in patients with cirrhosis.9 Therefore, patients at risk of HBV infection should be screened for it before starting these therapies.4 Furthermore, perhaps all patients about to undergo anticancer therapies that include anti-B-cell or anti-T-cell therapies or hematopoietic stem cell transplantation should be screened.9
Recent data indicate that many oncologists have not been screening for HBV.10 Hence, more effort is needed to make this important testing routine in this setting.
The initial tests in these patients should be liver chemistry tests, HBV surface antigen, HBV surface antibody, and HBV core antibody. In those who test positive for surface antigen, one should test for e antigen, e antibody, and HBV DNA.
Patients with indications for anti-HBV therapy (Table 1) should receive antiviral therapy. Otherwise, those positive for surface antigen should start taking anti-HBV medication at the start of chemotherapy or immunosuppressive therapy and should continue taking it until 6 months after the chemotherapy or immunosuppressive therapy is finished.4 Some experts also recommend starting anti-HBV therapy 7 days before the chemotherapy or immunosuppressive therapy and continuing it for 1 year afterward.10 Those with HBV DNA levels higher than 2 × 103 IU/mL should continue HBV therapy until they reach the same treatment end points as for immunocompetent patients as outlined above.4
Because we have little information on patients who are negative for surface antigen and who have antibodies against surface antigen and core antigen, we cannot make an unequivocal recommendation for anti-HBV therapy in this group.11 Rather, these patients should be monitored during immunosuppressive treatment, preferably with liver chemistry tests and HBV DNA titers, and antiviral drugs should be given as a deferred therapy upon evidence of HBV reactivation.9 Few cases of fatal hepatic failure in patients with this serologic pattern receiving rituximab (Rituxan) have been reported.12–14
With their small risk of drug resistance and rapid onset of action, entecavir or tenofovir may be the preferred anti-HBV therapy in patients undergoing immunosuppression or chemotherapy, especially in those requiring prolonged immunosuppressive therapy (longer than 12 months). In those requiring shorter courses, lamivudine or telbivudine is a possible alternative.4
OUTCOMES OF LIVER TRANSPLANTATION HAVE IMPROVED IN HBV PATIENTS
The early results of liver transplantation for HBV were discouraging because many patients developed rapidly progressive recurrent disease (fibrosing cholestatic hepatitis) and died within 12 to 18 months after the operation.15 However, patients with HBV are now treated perioperatively with lamivudine or adefovir combined with prolonged administration of hepatitis B immune globulin, and their survival now exceeds that of patients who receive transplants for many other conditions.16
Like patients with cirrhosis due to other causes, those with HBV-related cirrhosis who have any of the following should be referred for liver transplantation evaluation16:
- A Child-Turcotte-Pugh score of 7 or higher (Table 5).
- A major complication of cirrhosis such as ascites, variceal bleeding, hepatocellular carcinoma, or hepatic encephalopathy.
PREVENTING VERTICAL TRANSMISSION
The major problem in young women with chronic HBV infection is the risk of vertical (mother-to-infant) transmission at delivery. The risk varies, depending on the viral load and e antigen status of the mother at the time of delivery; if she is positive for e antigen, the risk of HBV infection in the newborn is 70% to 90% by the age of 6 months if the newborn does not receive postexposure immunoprophylaxis; if the mother is positive for surface antigen but negative for e antigen, the risk of chronic infection is less than 10%, even without postexposure immunoprophylaxis.17
All women should be tested for HBV surface antigen early in pregnancy each time they become pregnant. If a patient tests negative early in pregnancy but continues behaviors that put her at risk of HBV infection (eg, having multiple sexual partners, having had a sex partner positive for surface antigen, using injection drugs, or contracting any sexually transmitted disease), she should be retested at the time of admission to the hospital for delivery.17 This also includes women who were not screened prenatally and those with clinical hepatitis.
Vaccine and immune globulin for the infant
If the mother is positive for HBV surface antigen, the infant should receive single-antigen HBV vaccine and hepatitis B immune globulin within 12 hours of birth, given at different injection sites.17 The second dose of vaccine should be given at age 1 to 2 months and the third at age 6 months (but not before age 24 weeks). The response to vaccination should be ascertained by testing for surface antigen and surface antibody after completion of the vaccine series, at age 9 to 18 months.
Maternal HBV infection does not contraindicate breastfeeding, as studies suggest that breastfeeding by a mother positive for surface antigen does not increase the infant’s risk of acquiring HBV infection.18
Which HBV therapy for a pregnant woman?
Some evidence supports antiviral therapy with nucleoside/nucleotide analogues in pregnant women who have viral loads of 106 IU/mL or higher. Lamivudine is safe in pregnancy and, together with immunization of the infant, reduces HBV transmission. Interferon-based therapy is contraindicated in pregnant women (and in women who may want to become pregnant) because of interferon’s antiproliferative effects. Nucleoside/nucleotide analogues classified as category B (eg, lamivudine, telbivudine, and tenofovir) could be used when the benefit of treating the pregnant mother outweighs the risk to the mother or fetus,2 although the possible effects of tenofovir on bone density argue against its use during pregnancy or breastfeeding.19
VACCINATION HAS REDUCED THE INCIDENCE OF ACUTE HEPATITIS B
HBV vaccination, a major achievement in HBV management, has played a big role in reducing the incidence of acute HBV infection, especially in children and adolescents.20
The currently available vaccines in the United States contain HBV surface antigen derived through recombinant DNA technology from yeast.21 Two single-antigen vaccines are available in the United States, under the brand names Recombivax HB and Engerix B. Of the three licensed combination vaccines, one (Twinrix) is used in adults, and two (Comvax and Pediarix) are used in infants and young children. Twinrix contains recombinant HBV surface antigens and inactivated hepatitis A virus and it is recommended for people age 18 years and older and at risk of both HBV and hepatitis A infections.20
Vaccinate all infants
All infants should be vaccinated against HBV as part of the recommended childhood immunization schedule. The vaccine is given on a three-dose schedule at birth and again at 1 month and 6 months of age.16 All children and adolescents under age 19 who have not previously received HBV vaccine should be vaccinated at any age with an appropriate dose and schedule.16
Vaccinate adults at risk—or who ask for it
Vaccination is less effective in older people
The three-dose vaccine series given intramuscularly initially, then again at 1 month and 6 months, produces a protective antibody response in approximately 30% to 55% of healthy adults under age 40 after the first dose, 75% after the second dose, and more than 90% after the third dose.21,22 After age 40, however, the proportion of persons who have a protective antibody response after three doses declines to less than 90%, and by age 60, protective levels of antibody develop in only 75%.23
Other factors that lower the response to vaccination are smoking, obesity, genetic factors, and immune suppression.20
Postvaccination serologic testing for immunity is not necessary after routine vaccination of adults, but it is recommended for patients whose subsequent clinical management depends on knowledge of their immune status, such as health care workers who have contact with patients or blood and are at ongoing risk of injuries with sharp instruments or needlesticks; chronic hemodialysis patients and people infected with HIV or otherwise immunocompromised; and sex partners or needle-sharing partners of people positive for HBV surface antigen.20 A protective concentration of HBV surface antibody measured 1 to 2 months after completion of the vaccine series is defined as 10 mIU/mL. Further periodic testing to document persistence of protective levels of surface antibody is not indicated.
If the first series does not ‘take’
Patients who do not respond to the primary vaccine series should complete a second three-dose series, with doses at 0, 1, and 6 months. Serologic testing is done 1 to 2 months after finishing the second series.
Patients who do not have protective levels of HBV surface antibody after revaccination by the appropriate schedule in the deltoid muscle (< 5% of those receiving six doses of hepatitis B vaccine) either are primary nonresponders or are infected with HBV.20 Therefore, they should be tested for HBV surface antigen. If this test is negative, then they should be considered susceptible to HBV infection and should be counseled accordingly.
Contraindications and precautions
HBV vaccination is contraindicated in people with a history of hypersensitivity to baker’s yeast or to a previous dose of HBV vaccine.20 Patients with moderate or severe acute illness at the time the shot is scheduled should wait until they recover before getting HBV vaccine. Pregnancy is not a contraindication.20
- Elgouhari HM, Abu-Rajab Tamimi T, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 2008; 75:881–889.
- Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007; 35:2498–2508.
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007; 45:1056–1075.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004; 2:87–106.
- Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci 2005; 50:1525–1531.
- Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg–seropositive and 9421 HBsAg–seronegative male residents in Taiwan. Cancer Causes Control 2003; 14:241–250.
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236.
- Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006; 43:209–220.
- Tran T, Oh M, Poordad F, Martin P. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices [abstract]. Hepatology 2007; 46:978A.
- Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy–induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis 2007; 11:965–991.
- Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003; 102:1930.
- Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother 2005; 11:189–191.
- Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida FM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma 2005; 46:1085–1089.
- Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology 1991; 13:619–626.
- Murray KF, Carithers RLAASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast–feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975; 2:740–741.
- Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med 2005; 6:341–346.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1–33.
- Andre FE. Summary of safety and efficacy data on a yeast–derived hepatitis B vaccine. Am J Med 1989; 87:14S–20S.
- Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986; 13( suppl A):39–45.
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines: implications for persons at occupational risk for hepatitis B virus infection. Am J Prev Med 1998; 15:1–8.
Hepatitis b virus (HBV) infection is sometimes challenging to manage because the disease has several stages and many clinical scenarios. HBV-infected patients are a very heterogeneous group, and we cannot apply a single management approach to all.
An understanding of the natural history of HBV infection and its diagnosis, which we reviewed in last month’s issue of this Journal1, is critical to understanding how to manage HBV infection.
In this article, we will review the principles of HBV management in adults, including those on immunosuppressant therapy and pregnant women, and guidelines for HBV vaccination.
WORKUP FOR HBV INFECTION
Once the diagnosis of HBV infection is made,1 a full management strategy should be formulated, as outlined below.
History
When and how did the patient acquire HBV? This information is important to know when making treatment decisions. For example, most acute, adult-onset cases (eg, acquired recently via sexual contact or parenteral drug abuse) resolve spontaneously within a few months, whereas most chronic cases (defined as being positive for HBV surface antigen for more than 6 months) were acquired at birth or in early childhood. Therefore, we should try to determine if the patient’s mother, siblings, household contacts, and sexual partners are positive for HBV surface antigen or have risk factors for HBV infection1; those without infection or immunity to HBV should be vaccinated.
People at risk of HBV infection include:
- Parenteral drug users
- People with multiple sexual partners
- Household contacts and sexual partners of people positive for HBV surface antigen
- Infants born to HBV-infected mothers
- Patients and staff in custodial institutions for the developmentally disabled
- Recipients of certain plasma-derived products (including patients with congenital coagulation defects)
- Hemodialysis patients
- Health and public-safety workers who have contact with blood
- People born in areas where HBV is endemic, and their children.1
Does the patient have risk factors for other infections? Especially look for risk factors for human immunodeficiency virus (HIV) infection (eg, intravenous drug users and men having sex with men) and hepatitis D virus (intravenous drug users and patients from countries where hepatitis D virus infection is common, particularly Eastern Europe, Mediterranean countries, and the Amazon basin).
Does the patient have other modifiable risk factors for progressive liver disease, particularly alcohol abuse and obesity?
Does the patient have symptoms or signs of cirrhosis or hepatocellular carcinoma? Symptoms and signs that involve multiple systems could be extrahepatic manifestations of HBV infection, such as polyarteritis nodosa, which causes abdominal pain, arthralgia, hypertension, and asymmetric polyneuropathy.
Baseline laboratory evaluation
At baseline we should obtain a complete blood count, blood urea nitrogen level, serum creatinine level, liver profile, prothrombin time, urinalysis, and HBV serologic markers. In addition, HBV DNA can be detected in the serum at levels as low as 60 IU/mL, and it should be measured in the initial evaluation to establish a baseline before starting antiviral therapy in patients with chronic HBV infection and subsequently to monitor the response.
All patients with chronic HBV infection should also be tested for serologic markers of hepatitis A and hepatitis C; patients at risk of HIV and hepatitis D should also be tested for these diseases.
Not all patients need liver biopsy
Liver biopsy is the most accurate tool for staging the degree of HBV-related hepatic fibrosis in patients who have no obvious clinical manifestations of cirrhosis.
Not all patients with HBV infection need a biopsy, however. In patients with acute HBV infection, liver biopsy has no benefit except if concomitant pathology (eg, iron overload, nonalcoholic steatohepatitis, or alcoholic steatohepatitis) is suspected. In patients with chronic hepatitis B, liver biopsy is helpful when the viral load alone does not provide sufficient guidance for treatment, eg, when the viral load is less than 2 × 104 IU/mL in a patient positive for hepatitis e antigen or less than 2 × 103 IU/mL in a patient negative for hepatitis e antigen. (The presence of e antigen is a marker of HBV replication and infectivity.1) Biopsy should also be considered in those who have been infected a long time (eg, more than 10 years), because they may have occult cirrhosis, and if they do they may need to undergo antiviral treatment, endoscopy to look for varices, and surveillance for liver cancer.
In some situations it is easy to decide whether antiviral therapy is indicated without resorting to liver biopsy.
We would treat:
- A patient positive for HBV e antigen for more than 6 months, whose HBV DNA level is higher than 2 × 104 IU/mL and whose alanine aminotransferase (ALT) level is high
- A patient with HBV for more than 6 months who is negative for e antigen and who has an HBV DNA level higher than 2 × 103 IU/mL and elevated ALT
- A patient with compensated HBV cirrhosis and an HBV DNA level higher than 2 × 103 IU/mL
- A patient with HBV cirrhosis with decompensation and any detectable HBV DNA.
We would not treat:
- An HBV carrier with a normal ALT level and an HBV DNA level that is lower than 2 × 104 IU/mL or undetectable.
If a patient does not fit into one of these categories but has HBV DNA, a liver biopsy showing significant necroinflammation or fibrosis would be an indication for treatment.
ANTIVIRAL THERAPY
Below, we summarize the main principles of anti-HBV therapy, emphasizing whether to treat and with which agent. Treatment of HBV infection in patients who are also infected with HIV or hepatitis C virus and in those with resistant or refractory hepatitis B is not within the scope of this article.
Acute infection rarely needs treatment
Acute, adult-acquired HBV infection is self-limited in most cases,1 and antiviral therapy is not routinely indicated.
In the rare cases of acute liver failure related to acute HBV infection, use of a nucleoside or nucleotide analogue reverse transcriptase inhibitor (nucleoside/nucleotide analogues) has been recommended, although no properly designed studies have been done.2,3 This recommendation is based on anecdotal experience, the relative safety of the antiviral agents, the serious nature of acute liver failure, and the possible need for emergency liver transplantation that requires prophylaxis against recurrence.
The nucleoside/nucleotide analogues that have been recommended in acute liver failure are lamivudine (Epivir), telbivudine (Tyzeka), and entecavir (Baraclude)—but not adefovir (Hepsera), which has a slow action and potential nephrotoxicity. Interferon drugs are contraindicated because they frequently cause side effects and can worsen hepatitis.4
Patients with acute liver failure should be referred promptly to a liver transplant center, and other management measures should also be started in a timely fashion.
In chronic HBV infection, treatment decisions are individualized
In chronic hepatitis B (ie, lasting > 6 months), treatment decisions should be based on the patient’s clinical situation and test results. The route and duration of infection (if known), history of previous hepatitis flares, ALT levels, current and previous HBV serologic test results and DNA levels, findings on liver biopsy (if previously done), and clinically suspected cirrhosis are all important to consider when deciding whether antiviral therapy is needed.
For many patients with chronic HBV infection, observation without antiviral therapy is warranted, eg:
- Young patients (< 30 years old) who acquired HBV at birth and who have persistently normal ALT levels with no evidence of advanced liver disease, regardless of their HBV DNA level (immune tolerance phase)
- Chronic inactive carriers who have no e antigen, persistently normal ALT levels, and very low or undetectable levels of HBV DNA without evidence of significant liver injury.
These patients can be managed by internists by close monitoring for hepatitis flares with serial ALT measurements along with other general management measures.
Antiviral agents for chronic HBV infection
An ideal agent for treating hepatitis B does not exist. Trade-offs are the essence of agent selection.
Interferons, the first drugs shown to be effective against HBV, can in some respects be considered the best available initial choice, especially in patients positive for hepatitis e antigen. Interferons have numerous side effects but, unlike all the other options, they have a well-defined duration of treatment (4–6 months in patients positive for e antigen). The principal goal of this therapy is disappearance of e antigen.
Interferon-based therapy is not recommended in patients with cirrhosis, however, because of the risk of hepatic decompensation associated with interferon-related flares of hepatitis.4
Although single-agent antiviral therapy may someday be replaced by a multidrug regimen, the data so far are not sufficiently robust to recommend multidrug regimens except possibly in cases of established drug resistance.
Adjunctive management
Vaccinations. All patients with chronic hepatitis B should be vaccinated against hepatitis A if serologic testing indicates they have no immunity to it. Influenza and pneumococcal vaccines are recommended for all patients with chronic liver disease.6
Alcohol rehabilitation. Patients who abuse alcohol should be counseled, and many need consultation with a psychosocial care provider for alcohol rehabilitation.
Smoking cessation. Cigarette smoking is linked to a higher risk of hepatocellular carcinoma in patients with chronic liver disease, including chronic HBV infection.7 Therefore, smokers should be counseled to quit.
Surveillance for hepatocellular carcinoma. Hepatocellular carcinoma can occur in patients with chronic hepatitis B, in most cases on top of cirrhosis, although important exceptions exist. The American Association for the Study of Liver Diseases recommends surveillance for hepatocellular carcinoma in all HBV carriers with cirrhosis and in the following groups regardless of whether they have cirrhosis8:
- Asian men age 40 and older
- Asian women age 50 and older
- African patients age 20 and older
- Patients with a family history of hepatocellular carcinoma
- Possibly, those with high HBV DNA levels and ongoing inflammatory activity.
In the United States, liver ultrasonography and alpha fetoprotein measurement every 6 to 12 months is a reasonable strategy.
If there is evidence of cirrhosis, esophagogastroduodenoscopy is recommended to screen for esophageal and gastric varices.
SCREEN BEFORE CHEMOTHERAPY OR IMMUNOSUPPRESSIVE THERAPY
When patients who are positive for HBV surface antigen undergo immunosuppressive therapy or cancer chemotherapy, from 20% to 50% develop reactivated HBV infection with high HBV viral loads. Even patients who have resolved hepatitis B (ie, negative for HBV surface antigen and positive for surface antibody) may experience hepatitis B reactivation, with serious consequences. Hepatic decompensation and death have been reported during and after chemotherapy, especially in patients with cirrhosis.9 Therefore, patients at risk of HBV infection should be screened for it before starting these therapies.4 Furthermore, perhaps all patients about to undergo anticancer therapies that include anti-B-cell or anti-T-cell therapies or hematopoietic stem cell transplantation should be screened.9
Recent data indicate that many oncologists have not been screening for HBV.10 Hence, more effort is needed to make this important testing routine in this setting.
The initial tests in these patients should be liver chemistry tests, HBV surface antigen, HBV surface antibody, and HBV core antibody. In those who test positive for surface antigen, one should test for e antigen, e antibody, and HBV DNA.
Patients with indications for anti-HBV therapy (Table 1) should receive antiviral therapy. Otherwise, those positive for surface antigen should start taking anti-HBV medication at the start of chemotherapy or immunosuppressive therapy and should continue taking it until 6 months after the chemotherapy or immunosuppressive therapy is finished.4 Some experts also recommend starting anti-HBV therapy 7 days before the chemotherapy or immunosuppressive therapy and continuing it for 1 year afterward.10 Those with HBV DNA levels higher than 2 × 103 IU/mL should continue HBV therapy until they reach the same treatment end points as for immunocompetent patients as outlined above.4
Because we have little information on patients who are negative for surface antigen and who have antibodies against surface antigen and core antigen, we cannot make an unequivocal recommendation for anti-HBV therapy in this group.11 Rather, these patients should be monitored during immunosuppressive treatment, preferably with liver chemistry tests and HBV DNA titers, and antiviral drugs should be given as a deferred therapy upon evidence of HBV reactivation.9 Few cases of fatal hepatic failure in patients with this serologic pattern receiving rituximab (Rituxan) have been reported.12–14
With their small risk of drug resistance and rapid onset of action, entecavir or tenofovir may be the preferred anti-HBV therapy in patients undergoing immunosuppression or chemotherapy, especially in those requiring prolonged immunosuppressive therapy (longer than 12 months). In those requiring shorter courses, lamivudine or telbivudine is a possible alternative.4
OUTCOMES OF LIVER TRANSPLANTATION HAVE IMPROVED IN HBV PATIENTS
The early results of liver transplantation for HBV were discouraging because many patients developed rapidly progressive recurrent disease (fibrosing cholestatic hepatitis) and died within 12 to 18 months after the operation.15 However, patients with HBV are now treated perioperatively with lamivudine or adefovir combined with prolonged administration of hepatitis B immune globulin, and their survival now exceeds that of patients who receive transplants for many other conditions.16
Like patients with cirrhosis due to other causes, those with HBV-related cirrhosis who have any of the following should be referred for liver transplantation evaluation16:
- A Child-Turcotte-Pugh score of 7 or higher (Table 5).
- A major complication of cirrhosis such as ascites, variceal bleeding, hepatocellular carcinoma, or hepatic encephalopathy.
PREVENTING VERTICAL TRANSMISSION
The major problem in young women with chronic HBV infection is the risk of vertical (mother-to-infant) transmission at delivery. The risk varies, depending on the viral load and e antigen status of the mother at the time of delivery; if she is positive for e antigen, the risk of HBV infection in the newborn is 70% to 90% by the age of 6 months if the newborn does not receive postexposure immunoprophylaxis; if the mother is positive for surface antigen but negative for e antigen, the risk of chronic infection is less than 10%, even without postexposure immunoprophylaxis.17
All women should be tested for HBV surface antigen early in pregnancy each time they become pregnant. If a patient tests negative early in pregnancy but continues behaviors that put her at risk of HBV infection (eg, having multiple sexual partners, having had a sex partner positive for surface antigen, using injection drugs, or contracting any sexually transmitted disease), she should be retested at the time of admission to the hospital for delivery.17 This also includes women who were not screened prenatally and those with clinical hepatitis.
Vaccine and immune globulin for the infant
If the mother is positive for HBV surface antigen, the infant should receive single-antigen HBV vaccine and hepatitis B immune globulin within 12 hours of birth, given at different injection sites.17 The second dose of vaccine should be given at age 1 to 2 months and the third at age 6 months (but not before age 24 weeks). The response to vaccination should be ascertained by testing for surface antigen and surface antibody after completion of the vaccine series, at age 9 to 18 months.
Maternal HBV infection does not contraindicate breastfeeding, as studies suggest that breastfeeding by a mother positive for surface antigen does not increase the infant’s risk of acquiring HBV infection.18
Which HBV therapy for a pregnant woman?
Some evidence supports antiviral therapy with nucleoside/nucleotide analogues in pregnant women who have viral loads of 106 IU/mL or higher. Lamivudine is safe in pregnancy and, together with immunization of the infant, reduces HBV transmission. Interferon-based therapy is contraindicated in pregnant women (and in women who may want to become pregnant) because of interferon’s antiproliferative effects. Nucleoside/nucleotide analogues classified as category B (eg, lamivudine, telbivudine, and tenofovir) could be used when the benefit of treating the pregnant mother outweighs the risk to the mother or fetus,2 although the possible effects of tenofovir on bone density argue against its use during pregnancy or breastfeeding.19
VACCINATION HAS REDUCED THE INCIDENCE OF ACUTE HEPATITIS B
HBV vaccination, a major achievement in HBV management, has played a big role in reducing the incidence of acute HBV infection, especially in children and adolescents.20
The currently available vaccines in the United States contain HBV surface antigen derived through recombinant DNA technology from yeast.21 Two single-antigen vaccines are available in the United States, under the brand names Recombivax HB and Engerix B. Of the three licensed combination vaccines, one (Twinrix) is used in adults, and two (Comvax and Pediarix) are used in infants and young children. Twinrix contains recombinant HBV surface antigens and inactivated hepatitis A virus and it is recommended for people age 18 years and older and at risk of both HBV and hepatitis A infections.20
Vaccinate all infants
All infants should be vaccinated against HBV as part of the recommended childhood immunization schedule. The vaccine is given on a three-dose schedule at birth and again at 1 month and 6 months of age.16 All children and adolescents under age 19 who have not previously received HBV vaccine should be vaccinated at any age with an appropriate dose and schedule.16
Vaccinate adults at risk—or who ask for it
Vaccination is less effective in older people
The three-dose vaccine series given intramuscularly initially, then again at 1 month and 6 months, produces a protective antibody response in approximately 30% to 55% of healthy adults under age 40 after the first dose, 75% after the second dose, and more than 90% after the third dose.21,22 After age 40, however, the proportion of persons who have a protective antibody response after three doses declines to less than 90%, and by age 60, protective levels of antibody develop in only 75%.23
Other factors that lower the response to vaccination are smoking, obesity, genetic factors, and immune suppression.20
Postvaccination serologic testing for immunity is not necessary after routine vaccination of adults, but it is recommended for patients whose subsequent clinical management depends on knowledge of their immune status, such as health care workers who have contact with patients or blood and are at ongoing risk of injuries with sharp instruments or needlesticks; chronic hemodialysis patients and people infected with HIV or otherwise immunocompromised; and sex partners or needle-sharing partners of people positive for HBV surface antigen.20 A protective concentration of HBV surface antibody measured 1 to 2 months after completion of the vaccine series is defined as 10 mIU/mL. Further periodic testing to document persistence of protective levels of surface antibody is not indicated.
If the first series does not ‘take’
Patients who do not respond to the primary vaccine series should complete a second three-dose series, with doses at 0, 1, and 6 months. Serologic testing is done 1 to 2 months after finishing the second series.
Patients who do not have protective levels of HBV surface antibody after revaccination by the appropriate schedule in the deltoid muscle (< 5% of those receiving six doses of hepatitis B vaccine) either are primary nonresponders or are infected with HBV.20 Therefore, they should be tested for HBV surface antigen. If this test is negative, then they should be considered susceptible to HBV infection and should be counseled accordingly.
Contraindications and precautions
HBV vaccination is contraindicated in people with a history of hypersensitivity to baker’s yeast or to a previous dose of HBV vaccine.20 Patients with moderate or severe acute illness at the time the shot is scheduled should wait until they recover before getting HBV vaccine. Pregnancy is not a contraindication.20
Hepatitis b virus (HBV) infection is sometimes challenging to manage because the disease has several stages and many clinical scenarios. HBV-infected patients are a very heterogeneous group, and we cannot apply a single management approach to all.
An understanding of the natural history of HBV infection and its diagnosis, which we reviewed in last month’s issue of this Journal1, is critical to understanding how to manage HBV infection.
In this article, we will review the principles of HBV management in adults, including those on immunosuppressant therapy and pregnant women, and guidelines for HBV vaccination.
WORKUP FOR HBV INFECTION
Once the diagnosis of HBV infection is made,1 a full management strategy should be formulated, as outlined below.
History
When and how did the patient acquire HBV? This information is important to know when making treatment decisions. For example, most acute, adult-onset cases (eg, acquired recently via sexual contact or parenteral drug abuse) resolve spontaneously within a few months, whereas most chronic cases (defined as being positive for HBV surface antigen for more than 6 months) were acquired at birth or in early childhood. Therefore, we should try to determine if the patient’s mother, siblings, household contacts, and sexual partners are positive for HBV surface antigen or have risk factors for HBV infection1; those without infection or immunity to HBV should be vaccinated.
People at risk of HBV infection include:
- Parenteral drug users
- People with multiple sexual partners
- Household contacts and sexual partners of people positive for HBV surface antigen
- Infants born to HBV-infected mothers
- Patients and staff in custodial institutions for the developmentally disabled
- Recipients of certain plasma-derived products (including patients with congenital coagulation defects)
- Hemodialysis patients
- Health and public-safety workers who have contact with blood
- People born in areas where HBV is endemic, and their children.1
Does the patient have risk factors for other infections? Especially look for risk factors for human immunodeficiency virus (HIV) infection (eg, intravenous drug users and men having sex with men) and hepatitis D virus (intravenous drug users and patients from countries where hepatitis D virus infection is common, particularly Eastern Europe, Mediterranean countries, and the Amazon basin).
Does the patient have other modifiable risk factors for progressive liver disease, particularly alcohol abuse and obesity?
Does the patient have symptoms or signs of cirrhosis or hepatocellular carcinoma? Symptoms and signs that involve multiple systems could be extrahepatic manifestations of HBV infection, such as polyarteritis nodosa, which causes abdominal pain, arthralgia, hypertension, and asymmetric polyneuropathy.
Baseline laboratory evaluation
At baseline we should obtain a complete blood count, blood urea nitrogen level, serum creatinine level, liver profile, prothrombin time, urinalysis, and HBV serologic markers. In addition, HBV DNA can be detected in the serum at levels as low as 60 IU/mL, and it should be measured in the initial evaluation to establish a baseline before starting antiviral therapy in patients with chronic HBV infection and subsequently to monitor the response.
All patients with chronic HBV infection should also be tested for serologic markers of hepatitis A and hepatitis C; patients at risk of HIV and hepatitis D should also be tested for these diseases.
Not all patients need liver biopsy
Liver biopsy is the most accurate tool for staging the degree of HBV-related hepatic fibrosis in patients who have no obvious clinical manifestations of cirrhosis.
Not all patients with HBV infection need a biopsy, however. In patients with acute HBV infection, liver biopsy has no benefit except if concomitant pathology (eg, iron overload, nonalcoholic steatohepatitis, or alcoholic steatohepatitis) is suspected. In patients with chronic hepatitis B, liver biopsy is helpful when the viral load alone does not provide sufficient guidance for treatment, eg, when the viral load is less than 2 × 104 IU/mL in a patient positive for hepatitis e antigen or less than 2 × 103 IU/mL in a patient negative for hepatitis e antigen. (The presence of e antigen is a marker of HBV replication and infectivity.1) Biopsy should also be considered in those who have been infected a long time (eg, more than 10 years), because they may have occult cirrhosis, and if they do they may need to undergo antiviral treatment, endoscopy to look for varices, and surveillance for liver cancer.
In some situations it is easy to decide whether antiviral therapy is indicated without resorting to liver biopsy.
We would treat:
- A patient positive for HBV e antigen for more than 6 months, whose HBV DNA level is higher than 2 × 104 IU/mL and whose alanine aminotransferase (ALT) level is high
- A patient with HBV for more than 6 months who is negative for e antigen and who has an HBV DNA level higher than 2 × 103 IU/mL and elevated ALT
- A patient with compensated HBV cirrhosis and an HBV DNA level higher than 2 × 103 IU/mL
- A patient with HBV cirrhosis with decompensation and any detectable HBV DNA.
We would not treat:
- An HBV carrier with a normal ALT level and an HBV DNA level that is lower than 2 × 104 IU/mL or undetectable.
If a patient does not fit into one of these categories but has HBV DNA, a liver biopsy showing significant necroinflammation or fibrosis would be an indication for treatment.
ANTIVIRAL THERAPY
Below, we summarize the main principles of anti-HBV therapy, emphasizing whether to treat and with which agent. Treatment of HBV infection in patients who are also infected with HIV or hepatitis C virus and in those with resistant or refractory hepatitis B is not within the scope of this article.
Acute infection rarely needs treatment
Acute, adult-acquired HBV infection is self-limited in most cases,1 and antiviral therapy is not routinely indicated.
In the rare cases of acute liver failure related to acute HBV infection, use of a nucleoside or nucleotide analogue reverse transcriptase inhibitor (nucleoside/nucleotide analogues) has been recommended, although no properly designed studies have been done.2,3 This recommendation is based on anecdotal experience, the relative safety of the antiviral agents, the serious nature of acute liver failure, and the possible need for emergency liver transplantation that requires prophylaxis against recurrence.
The nucleoside/nucleotide analogues that have been recommended in acute liver failure are lamivudine (Epivir), telbivudine (Tyzeka), and entecavir (Baraclude)—but not adefovir (Hepsera), which has a slow action and potential nephrotoxicity. Interferon drugs are contraindicated because they frequently cause side effects and can worsen hepatitis.4
Patients with acute liver failure should be referred promptly to a liver transplant center, and other management measures should also be started in a timely fashion.
In chronic HBV infection, treatment decisions are individualized
In chronic hepatitis B (ie, lasting > 6 months), treatment decisions should be based on the patient’s clinical situation and test results. The route and duration of infection (if known), history of previous hepatitis flares, ALT levels, current and previous HBV serologic test results and DNA levels, findings on liver biopsy (if previously done), and clinically suspected cirrhosis are all important to consider when deciding whether antiviral therapy is needed.
For many patients with chronic HBV infection, observation without antiviral therapy is warranted, eg:
- Young patients (< 30 years old) who acquired HBV at birth and who have persistently normal ALT levels with no evidence of advanced liver disease, regardless of their HBV DNA level (immune tolerance phase)
- Chronic inactive carriers who have no e antigen, persistently normal ALT levels, and very low or undetectable levels of HBV DNA without evidence of significant liver injury.
These patients can be managed by internists by close monitoring for hepatitis flares with serial ALT measurements along with other general management measures.
Antiviral agents for chronic HBV infection
An ideal agent for treating hepatitis B does not exist. Trade-offs are the essence of agent selection.
Interferons, the first drugs shown to be effective against HBV, can in some respects be considered the best available initial choice, especially in patients positive for hepatitis e antigen. Interferons have numerous side effects but, unlike all the other options, they have a well-defined duration of treatment (4–6 months in patients positive for e antigen). The principal goal of this therapy is disappearance of e antigen.
Interferon-based therapy is not recommended in patients with cirrhosis, however, because of the risk of hepatic decompensation associated with interferon-related flares of hepatitis.4
Although single-agent antiviral therapy may someday be replaced by a multidrug regimen, the data so far are not sufficiently robust to recommend multidrug regimens except possibly in cases of established drug resistance.
Adjunctive management
Vaccinations. All patients with chronic hepatitis B should be vaccinated against hepatitis A if serologic testing indicates they have no immunity to it. Influenza and pneumococcal vaccines are recommended for all patients with chronic liver disease.6
Alcohol rehabilitation. Patients who abuse alcohol should be counseled, and many need consultation with a psychosocial care provider for alcohol rehabilitation.
Smoking cessation. Cigarette smoking is linked to a higher risk of hepatocellular carcinoma in patients with chronic liver disease, including chronic HBV infection.7 Therefore, smokers should be counseled to quit.
Surveillance for hepatocellular carcinoma. Hepatocellular carcinoma can occur in patients with chronic hepatitis B, in most cases on top of cirrhosis, although important exceptions exist. The American Association for the Study of Liver Diseases recommends surveillance for hepatocellular carcinoma in all HBV carriers with cirrhosis and in the following groups regardless of whether they have cirrhosis8:
- Asian men age 40 and older
- Asian women age 50 and older
- African patients age 20 and older
- Patients with a family history of hepatocellular carcinoma
- Possibly, those with high HBV DNA levels and ongoing inflammatory activity.
In the United States, liver ultrasonography and alpha fetoprotein measurement every 6 to 12 months is a reasonable strategy.
If there is evidence of cirrhosis, esophagogastroduodenoscopy is recommended to screen for esophageal and gastric varices.
SCREEN BEFORE CHEMOTHERAPY OR IMMUNOSUPPRESSIVE THERAPY
When patients who are positive for HBV surface antigen undergo immunosuppressive therapy or cancer chemotherapy, from 20% to 50% develop reactivated HBV infection with high HBV viral loads. Even patients who have resolved hepatitis B (ie, negative for HBV surface antigen and positive for surface antibody) may experience hepatitis B reactivation, with serious consequences. Hepatic decompensation and death have been reported during and after chemotherapy, especially in patients with cirrhosis.9 Therefore, patients at risk of HBV infection should be screened for it before starting these therapies.4 Furthermore, perhaps all patients about to undergo anticancer therapies that include anti-B-cell or anti-T-cell therapies or hematopoietic stem cell transplantation should be screened.9
Recent data indicate that many oncologists have not been screening for HBV.10 Hence, more effort is needed to make this important testing routine in this setting.
The initial tests in these patients should be liver chemistry tests, HBV surface antigen, HBV surface antibody, and HBV core antibody. In those who test positive for surface antigen, one should test for e antigen, e antibody, and HBV DNA.
Patients with indications for anti-HBV therapy (Table 1) should receive antiviral therapy. Otherwise, those positive for surface antigen should start taking anti-HBV medication at the start of chemotherapy or immunosuppressive therapy and should continue taking it until 6 months after the chemotherapy or immunosuppressive therapy is finished.4 Some experts also recommend starting anti-HBV therapy 7 days before the chemotherapy or immunosuppressive therapy and continuing it for 1 year afterward.10 Those with HBV DNA levels higher than 2 × 103 IU/mL should continue HBV therapy until they reach the same treatment end points as for immunocompetent patients as outlined above.4
Because we have little information on patients who are negative for surface antigen and who have antibodies against surface antigen and core antigen, we cannot make an unequivocal recommendation for anti-HBV therapy in this group.11 Rather, these patients should be monitored during immunosuppressive treatment, preferably with liver chemistry tests and HBV DNA titers, and antiviral drugs should be given as a deferred therapy upon evidence of HBV reactivation.9 Few cases of fatal hepatic failure in patients with this serologic pattern receiving rituximab (Rituxan) have been reported.12–14
With their small risk of drug resistance and rapid onset of action, entecavir or tenofovir may be the preferred anti-HBV therapy in patients undergoing immunosuppression or chemotherapy, especially in those requiring prolonged immunosuppressive therapy (longer than 12 months). In those requiring shorter courses, lamivudine or telbivudine is a possible alternative.4
OUTCOMES OF LIVER TRANSPLANTATION HAVE IMPROVED IN HBV PATIENTS
The early results of liver transplantation for HBV were discouraging because many patients developed rapidly progressive recurrent disease (fibrosing cholestatic hepatitis) and died within 12 to 18 months after the operation.15 However, patients with HBV are now treated perioperatively with lamivudine or adefovir combined with prolonged administration of hepatitis B immune globulin, and their survival now exceeds that of patients who receive transplants for many other conditions.16
Like patients with cirrhosis due to other causes, those with HBV-related cirrhosis who have any of the following should be referred for liver transplantation evaluation16:
- A Child-Turcotte-Pugh score of 7 or higher (Table 5).
- A major complication of cirrhosis such as ascites, variceal bleeding, hepatocellular carcinoma, or hepatic encephalopathy.
PREVENTING VERTICAL TRANSMISSION
The major problem in young women with chronic HBV infection is the risk of vertical (mother-to-infant) transmission at delivery. The risk varies, depending on the viral load and e antigen status of the mother at the time of delivery; if she is positive for e antigen, the risk of HBV infection in the newborn is 70% to 90% by the age of 6 months if the newborn does not receive postexposure immunoprophylaxis; if the mother is positive for surface antigen but negative for e antigen, the risk of chronic infection is less than 10%, even without postexposure immunoprophylaxis.17
All women should be tested for HBV surface antigen early in pregnancy each time they become pregnant. If a patient tests negative early in pregnancy but continues behaviors that put her at risk of HBV infection (eg, having multiple sexual partners, having had a sex partner positive for surface antigen, using injection drugs, or contracting any sexually transmitted disease), she should be retested at the time of admission to the hospital for delivery.17 This also includes women who were not screened prenatally and those with clinical hepatitis.
Vaccine and immune globulin for the infant
If the mother is positive for HBV surface antigen, the infant should receive single-antigen HBV vaccine and hepatitis B immune globulin within 12 hours of birth, given at different injection sites.17 The second dose of vaccine should be given at age 1 to 2 months and the third at age 6 months (but not before age 24 weeks). The response to vaccination should be ascertained by testing for surface antigen and surface antibody after completion of the vaccine series, at age 9 to 18 months.
Maternal HBV infection does not contraindicate breastfeeding, as studies suggest that breastfeeding by a mother positive for surface antigen does not increase the infant’s risk of acquiring HBV infection.18
Which HBV therapy for a pregnant woman?
Some evidence supports antiviral therapy with nucleoside/nucleotide analogues in pregnant women who have viral loads of 106 IU/mL or higher. Lamivudine is safe in pregnancy and, together with immunization of the infant, reduces HBV transmission. Interferon-based therapy is contraindicated in pregnant women (and in women who may want to become pregnant) because of interferon’s antiproliferative effects. Nucleoside/nucleotide analogues classified as category B (eg, lamivudine, telbivudine, and tenofovir) could be used when the benefit of treating the pregnant mother outweighs the risk to the mother or fetus,2 although the possible effects of tenofovir on bone density argue against its use during pregnancy or breastfeeding.19
VACCINATION HAS REDUCED THE INCIDENCE OF ACUTE HEPATITIS B
HBV vaccination, a major achievement in HBV management, has played a big role in reducing the incidence of acute HBV infection, especially in children and adolescents.20
The currently available vaccines in the United States contain HBV surface antigen derived through recombinant DNA technology from yeast.21 Two single-antigen vaccines are available in the United States, under the brand names Recombivax HB and Engerix B. Of the three licensed combination vaccines, one (Twinrix) is used in adults, and two (Comvax and Pediarix) are used in infants and young children. Twinrix contains recombinant HBV surface antigens and inactivated hepatitis A virus and it is recommended for people age 18 years and older and at risk of both HBV and hepatitis A infections.20
Vaccinate all infants
All infants should be vaccinated against HBV as part of the recommended childhood immunization schedule. The vaccine is given on a three-dose schedule at birth and again at 1 month and 6 months of age.16 All children and adolescents under age 19 who have not previously received HBV vaccine should be vaccinated at any age with an appropriate dose and schedule.16
Vaccinate adults at risk—or who ask for it
Vaccination is less effective in older people
The three-dose vaccine series given intramuscularly initially, then again at 1 month and 6 months, produces a protective antibody response in approximately 30% to 55% of healthy adults under age 40 after the first dose, 75% after the second dose, and more than 90% after the third dose.21,22 After age 40, however, the proportion of persons who have a protective antibody response after three doses declines to less than 90%, and by age 60, protective levels of antibody develop in only 75%.23
Other factors that lower the response to vaccination are smoking, obesity, genetic factors, and immune suppression.20
Postvaccination serologic testing for immunity is not necessary after routine vaccination of adults, but it is recommended for patients whose subsequent clinical management depends on knowledge of their immune status, such as health care workers who have contact with patients or blood and are at ongoing risk of injuries with sharp instruments or needlesticks; chronic hemodialysis patients and people infected with HIV or otherwise immunocompromised; and sex partners or needle-sharing partners of people positive for HBV surface antigen.20 A protective concentration of HBV surface antibody measured 1 to 2 months after completion of the vaccine series is defined as 10 mIU/mL. Further periodic testing to document persistence of protective levels of surface antibody is not indicated.
If the first series does not ‘take’
Patients who do not respond to the primary vaccine series should complete a second three-dose series, with doses at 0, 1, and 6 months. Serologic testing is done 1 to 2 months after finishing the second series.
Patients who do not have protective levels of HBV surface antibody after revaccination by the appropriate schedule in the deltoid muscle (< 5% of those receiving six doses of hepatitis B vaccine) either are primary nonresponders or are infected with HBV.20 Therefore, they should be tested for HBV surface antigen. If this test is negative, then they should be considered susceptible to HBV infection and should be counseled accordingly.
Contraindications and precautions
HBV vaccination is contraindicated in people with a history of hypersensitivity to baker’s yeast or to a previous dose of HBV vaccine.20 Patients with moderate or severe acute illness at the time the shot is scheduled should wait until they recover before getting HBV vaccine. Pregnancy is not a contraindication.20
- Elgouhari HM, Abu-Rajab Tamimi T, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 2008; 75:881–889.
- Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007; 35:2498–2508.
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007; 45:1056–1075.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004; 2:87–106.
- Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci 2005; 50:1525–1531.
- Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg–seropositive and 9421 HBsAg–seronegative male residents in Taiwan. Cancer Causes Control 2003; 14:241–250.
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236.
- Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006; 43:209–220.
- Tran T, Oh M, Poordad F, Martin P. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices [abstract]. Hepatology 2007; 46:978A.
- Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy–induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis 2007; 11:965–991.
- Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003; 102:1930.
- Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother 2005; 11:189–191.
- Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida FM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma 2005; 46:1085–1089.
- Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology 1991; 13:619–626.
- Murray KF, Carithers RLAASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast–feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975; 2:740–741.
- Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med 2005; 6:341–346.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1–33.
- Andre FE. Summary of safety and efficacy data on a yeast–derived hepatitis B vaccine. Am J Med 1989; 87:14S–20S.
- Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986; 13( suppl A):39–45.
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines: implications for persons at occupational risk for hepatitis B virus infection. Am J Prev Med 1998; 15:1–8.
- Elgouhari HM, Abu-Rajab Tamimi T, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 2008; 75:881–889.
- Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007; 35:2498–2508.
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007; 45:1056–1075.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004; 2:87–106.
- Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci 2005; 50:1525–1531.
- Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg–seropositive and 9421 HBsAg–seronegative male residents in Taiwan. Cancer Causes Control 2003; 14:241–250.
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236.
- Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006; 43:209–220.
- Tran T, Oh M, Poordad F, Martin P. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices [abstract]. Hepatology 2007; 46:978A.
- Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy–induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis 2007; 11:965–991.
- Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003; 102:1930.
- Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother 2005; 11:189–191.
- Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida FM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma 2005; 46:1085–1089.
- Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology 1991; 13:619–626.
- Murray KF, Carithers RLAASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31.
- Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast–feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975; 2:740–741.
- Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med 2005; 6:341–346.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1–33.
- Andre FE. Summary of safety and efficacy data on a yeast–derived hepatitis B vaccine. Am J Med 1989; 87:14S–20S.
- Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986; 13( suppl A):39–45.
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines: implications for persons at occupational risk for hepatitis B virus infection. Am J Prev Med 1998; 15:1–8.
KEY POINTS
- Patients with HBV infection should be screened for hepatocellular carcinoma, especially if they have cirrhosis.
- Nucleoside and nucleotide analogue reverse transcriptase inhibitors are easy to use and therefore are usually the first-line therapy. Problems with these agents are that the optimal treatment duration is not known, and that drug resistance can emerge.
- Patients with advanced liver disease or hepatocellular carcinoma should be referred promptly for possible liver transplantation.
- Candidates for immunosuppressant therapy or cytotoxic chemotherapy should be screened for HBV, as this therapy can cause a potentially fatal flare of HBV.
- People at risk should be vaccinated; many have not been.