User login
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.
"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
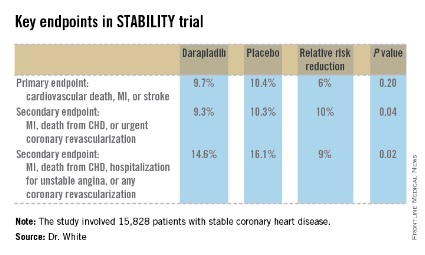
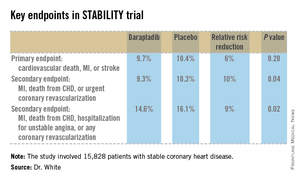
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.

"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.

"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
AT ACC 14
Major finding: The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. But a significant reduction was seen in major coronary events.
Data source: STABILITY, a randomized, double-blind study in which 15,828 patients with stable coronary heart disease were followed on darapladib or placebo for a median of 3.7 years.
Disclosures: STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.