User login
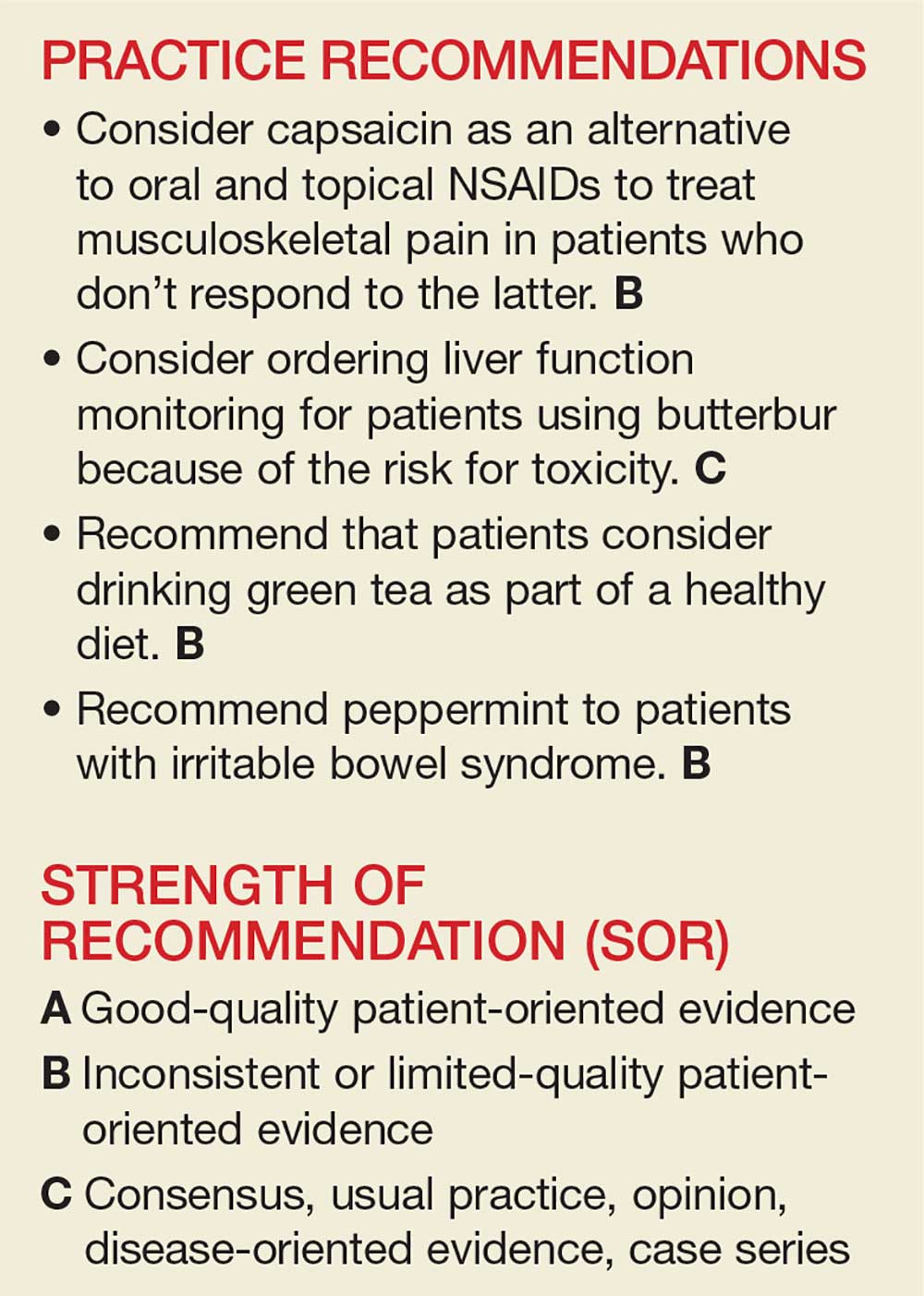
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
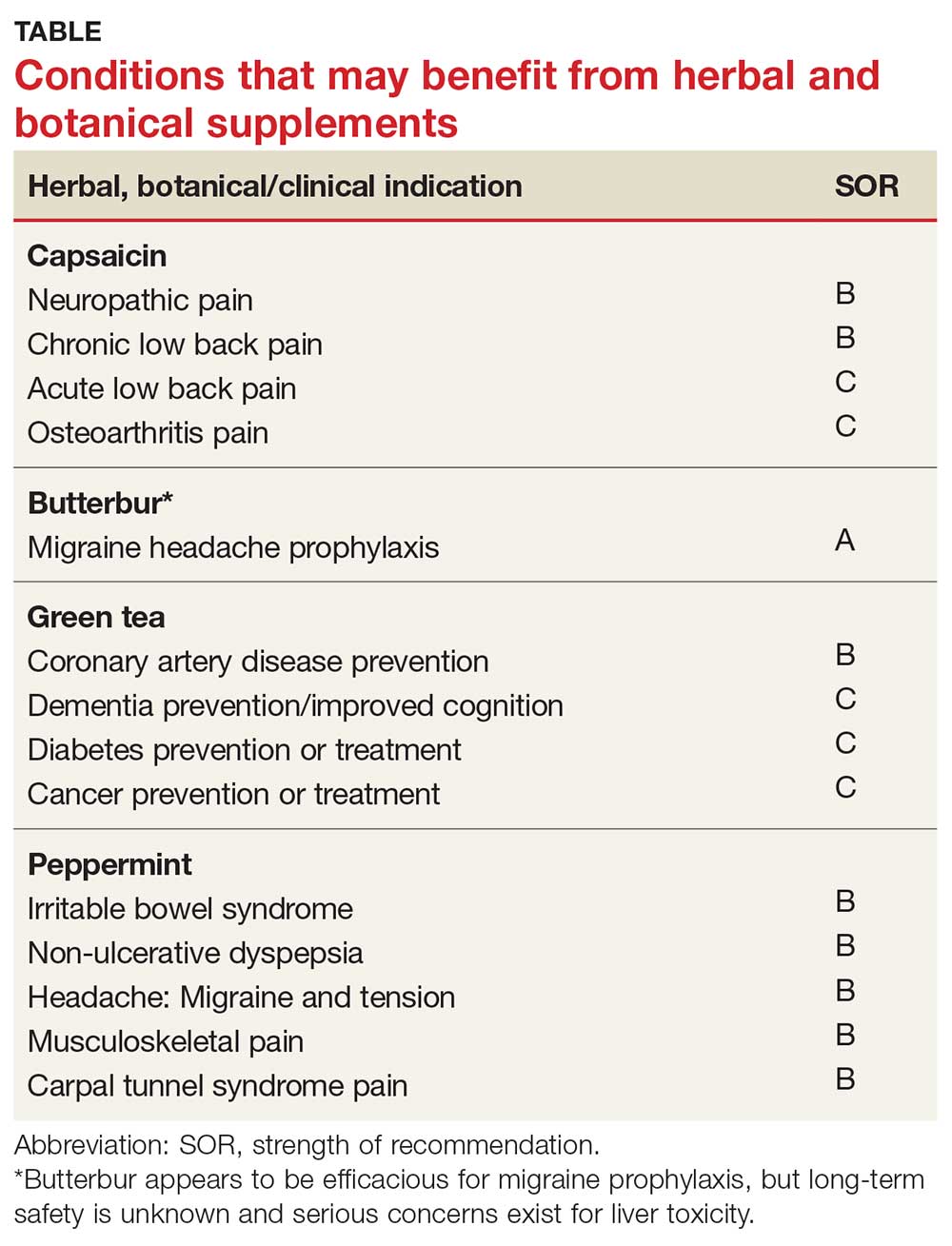
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.