User login
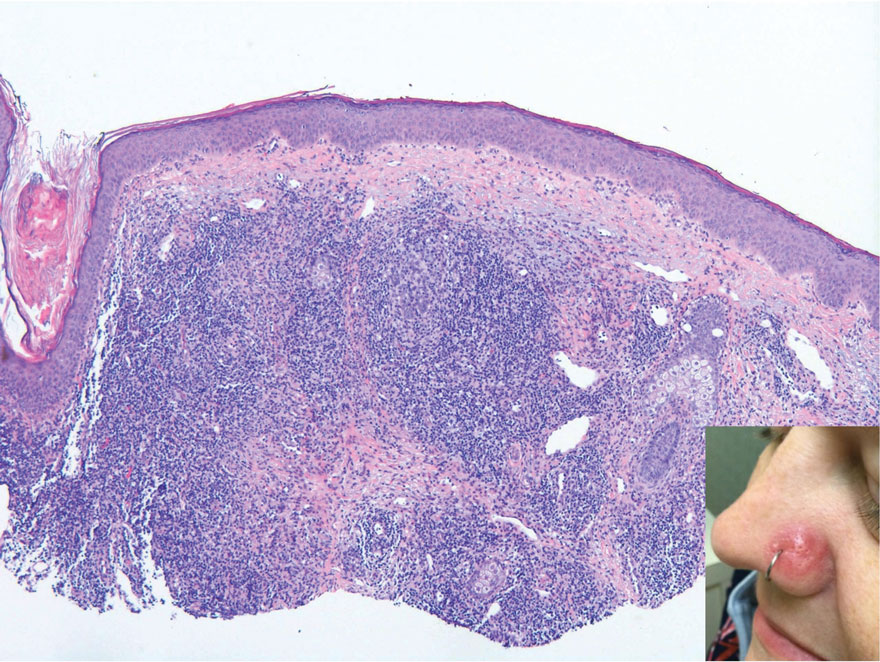
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
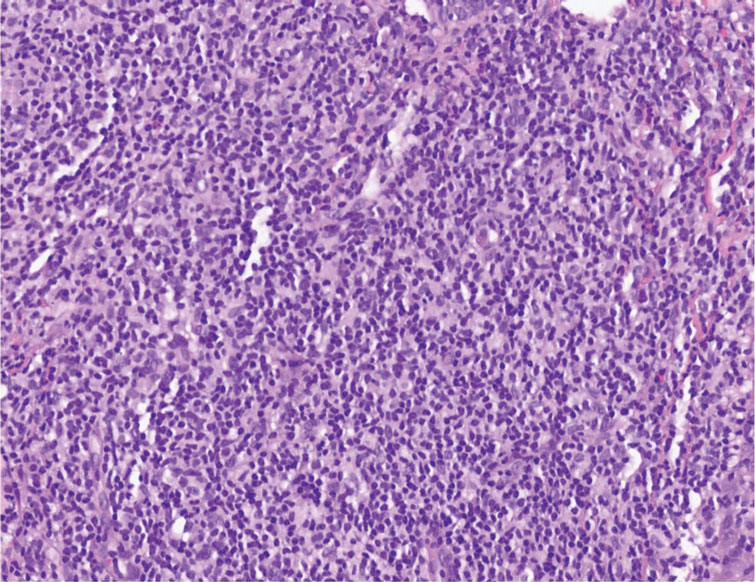
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
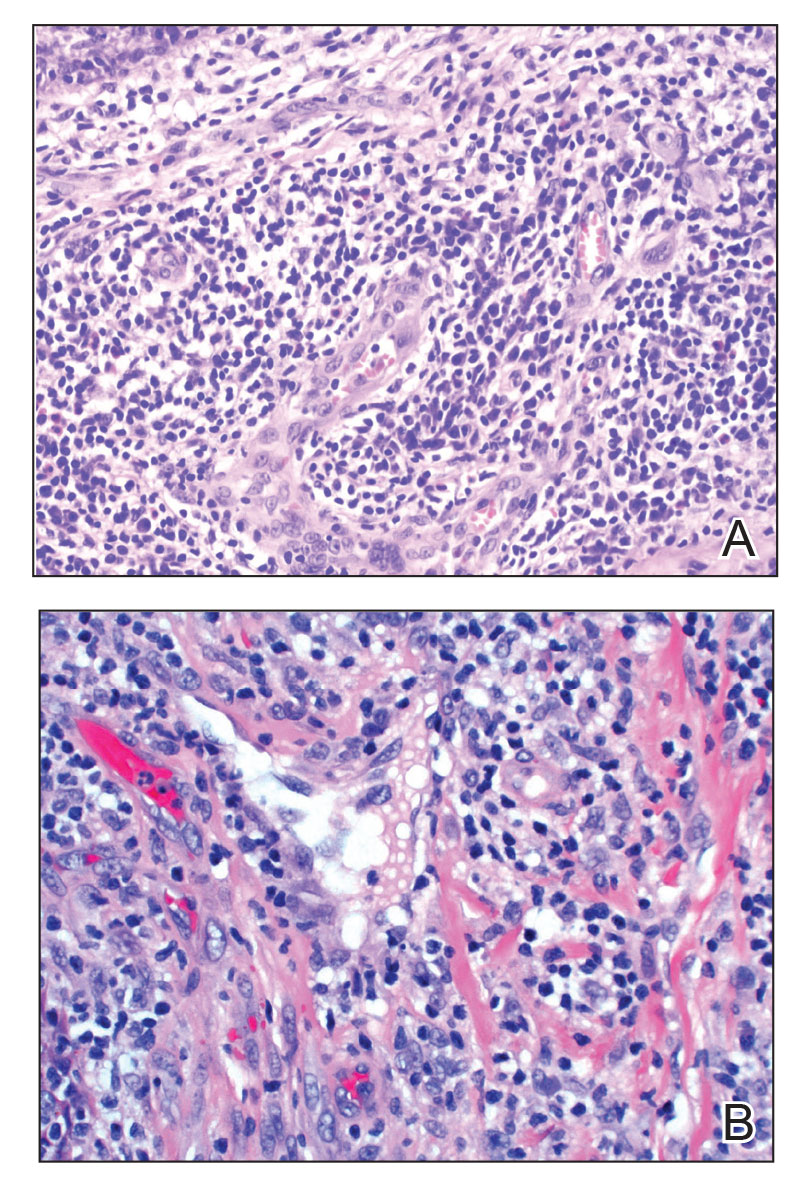
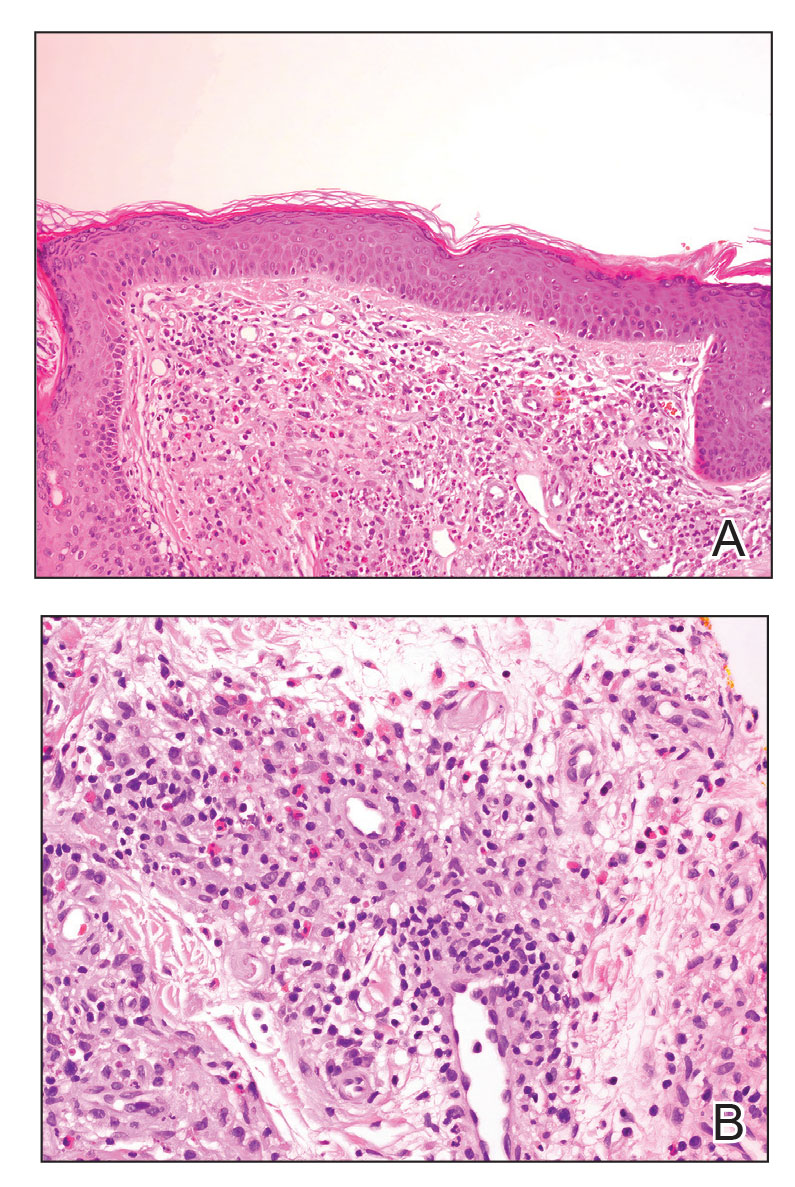
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.
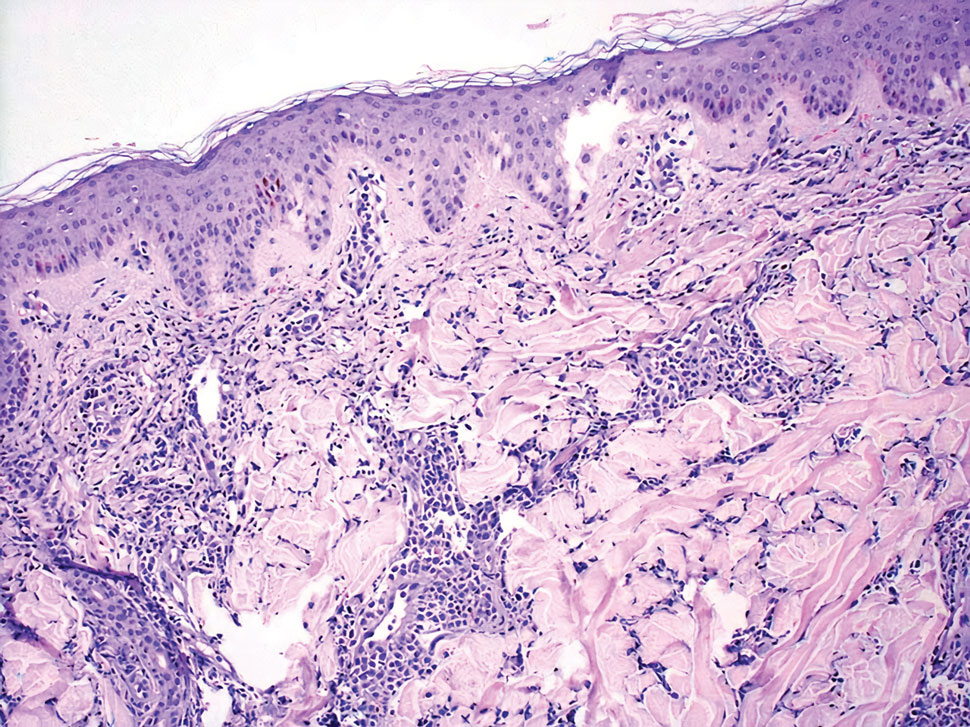
Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12
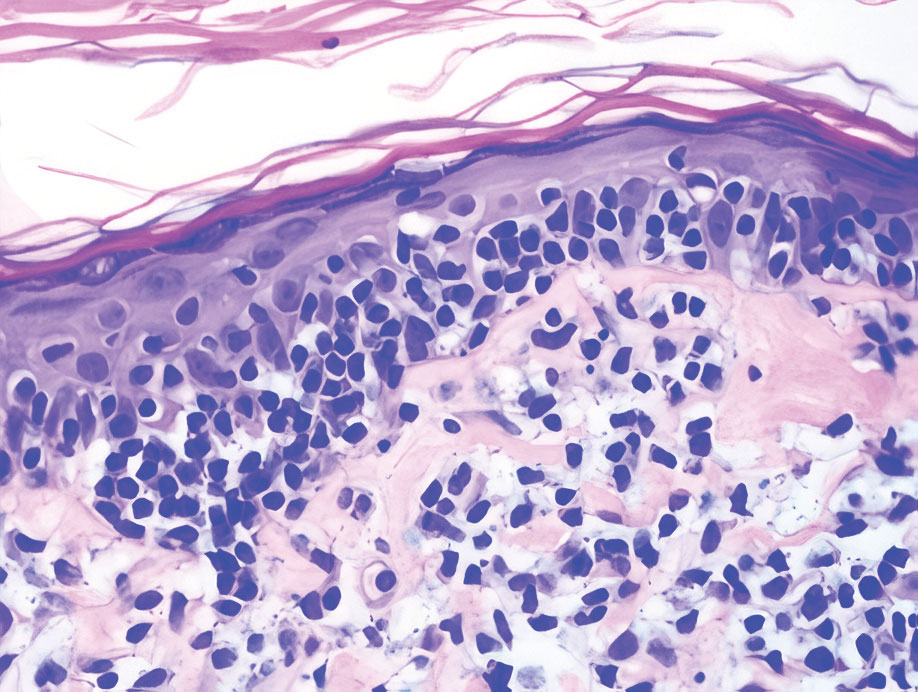
Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
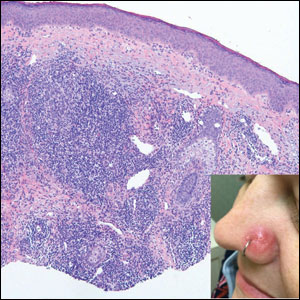
A 35-year-old woman presented with a slowly growing, smooth, erythematous papule of 2 months’ duration on the left nasal ala surrounding a piercing (top, inset) that had been performed 4 years prior. A tangential biopsy was obtained for histopathologic evaluation.