User login
Case Report
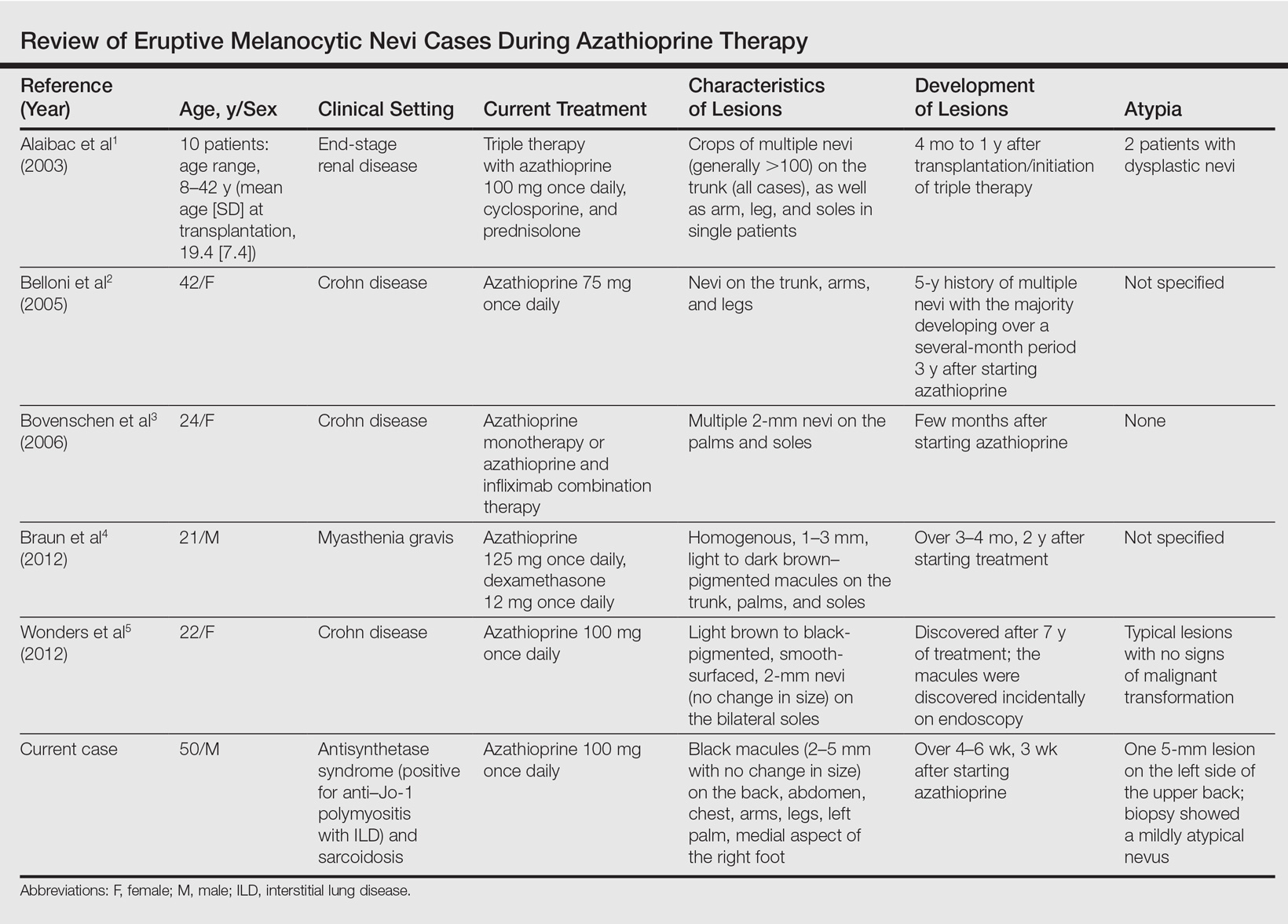
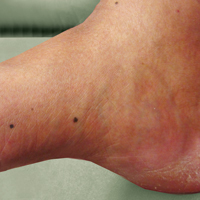
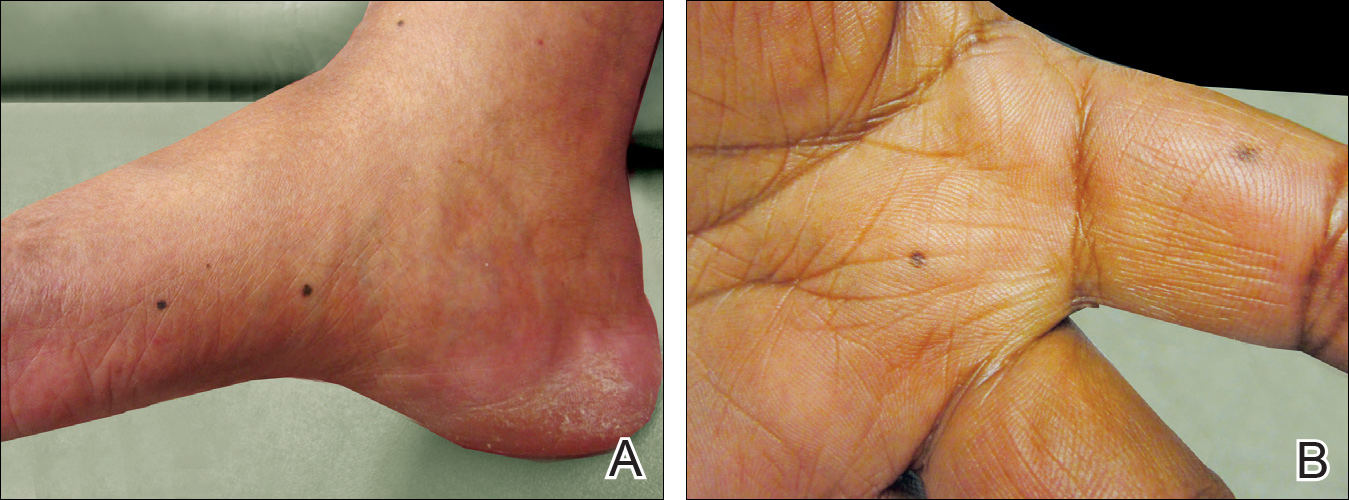
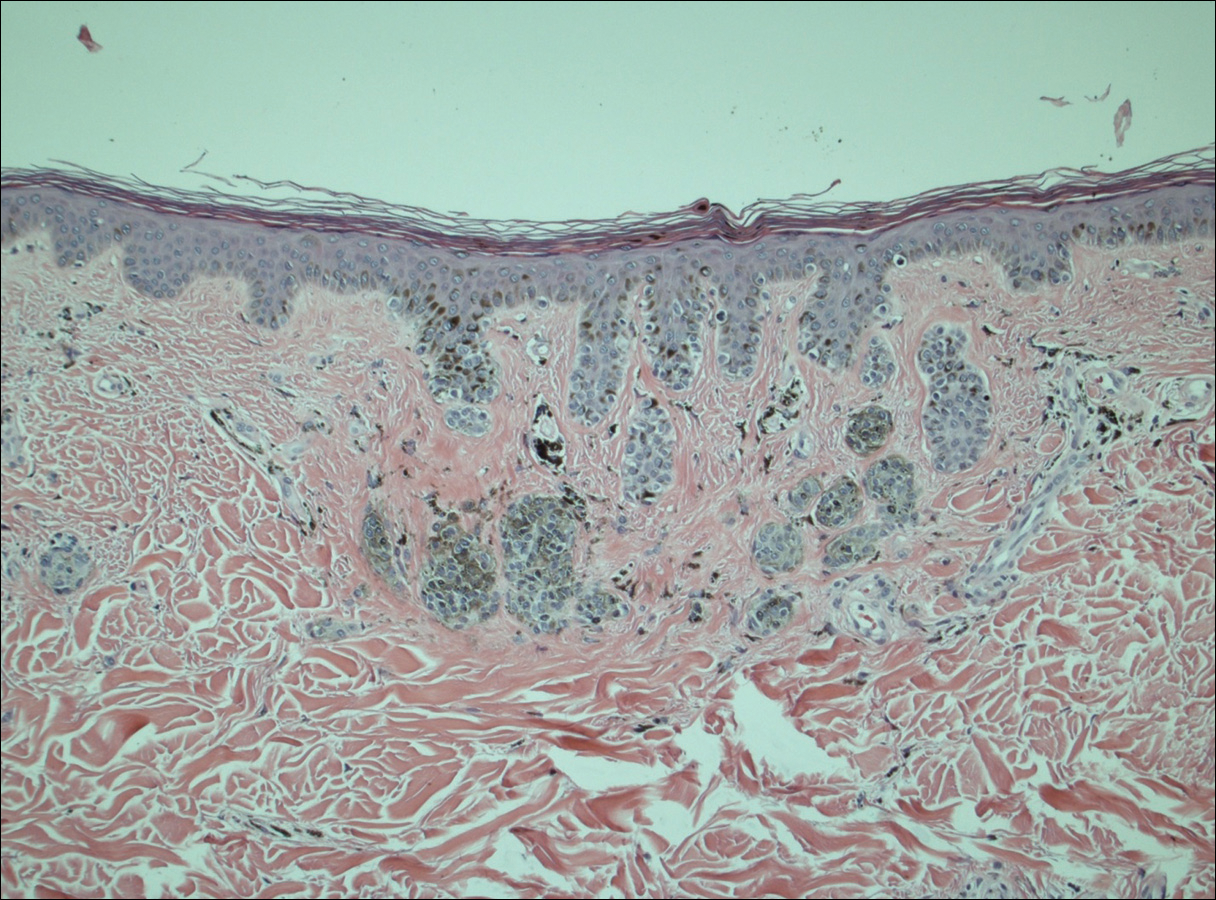
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.