User login
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
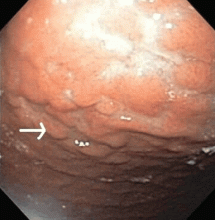
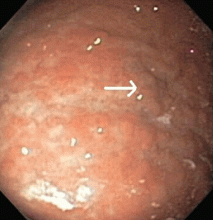
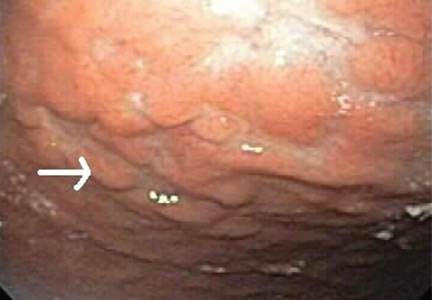
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
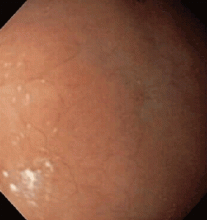
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.