User login
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
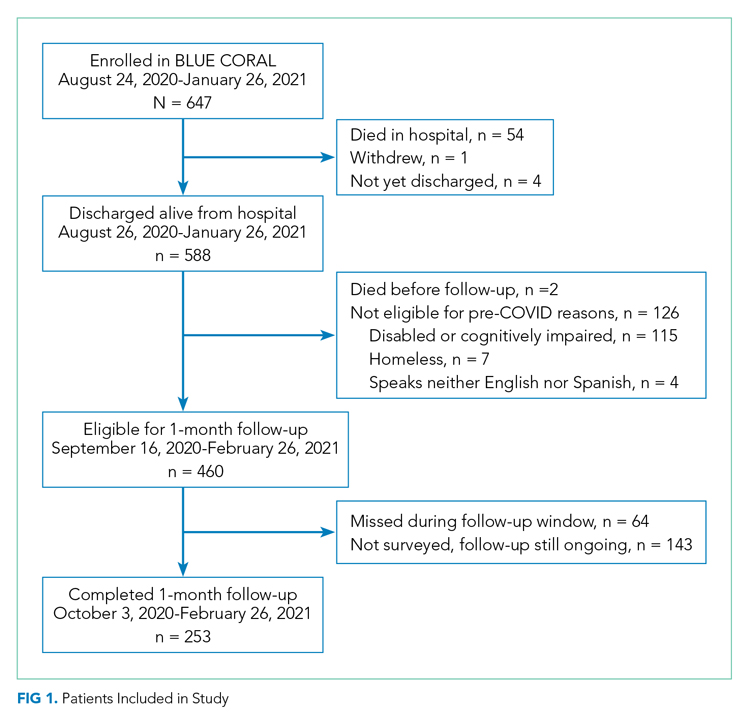
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.
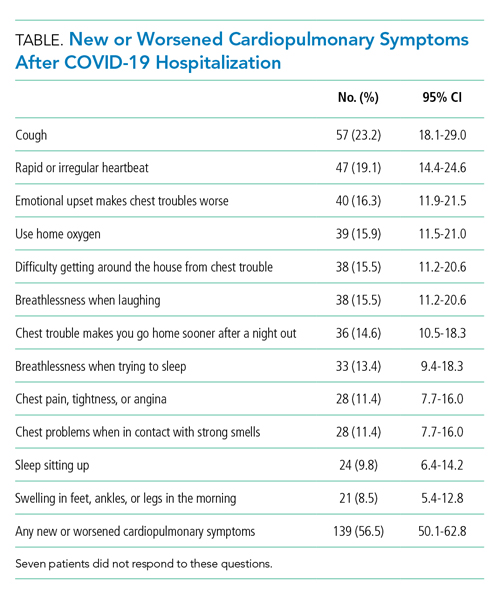
One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).
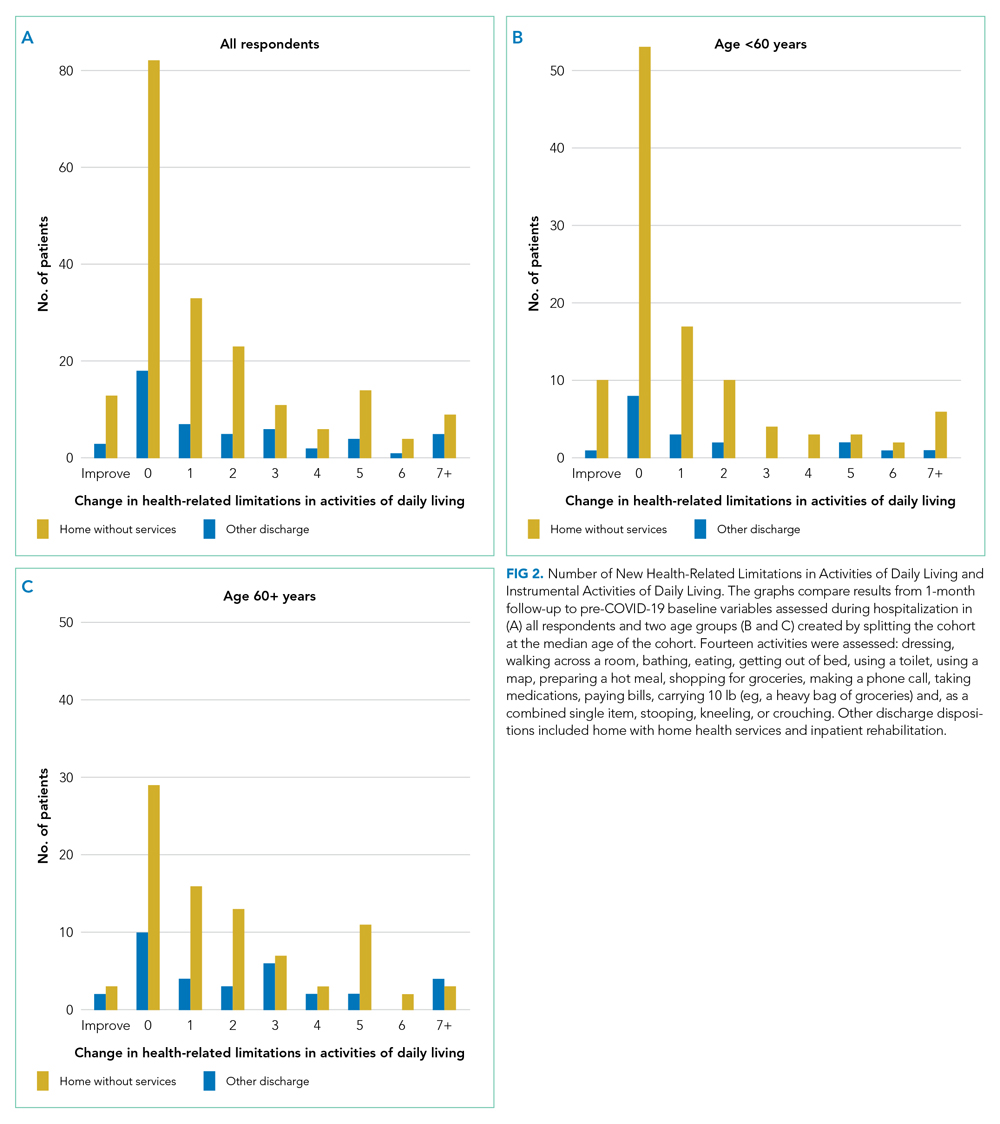
New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.
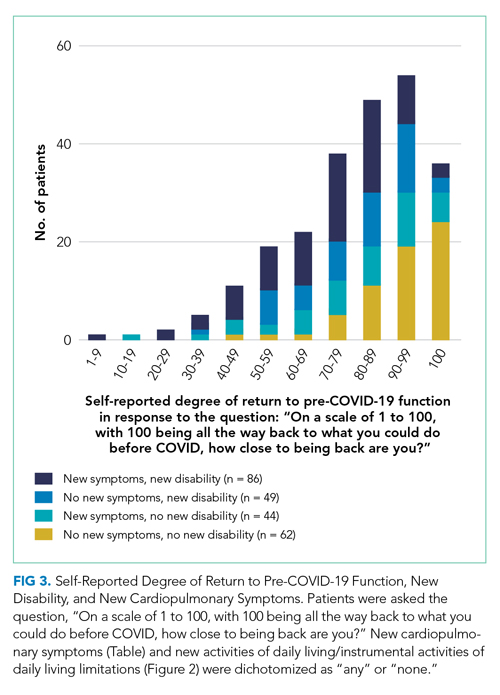
At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).
More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.
One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).
New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.
At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).
More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.
One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).
New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.
At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).
More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
© 2021 Society of Hospital Medicine