User login
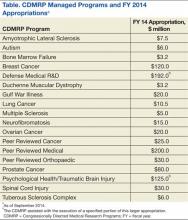
The Congressionally Directed Medical Research Programs (CDMRP), an office within the U.S. Army Medical Research and Materiel Command, has executed funding for research in 18 biomedical programs (Table). These programs touch the lives of service members, veterans, family members, and the general public. A partnership with the military, government, scientific community, survivors, patients, and their family members brings together spheres of stakeholders that typically might not otherwise collaborate and enables the CDMRP to complement other sources of research funding while focusing on research most directly relevant to each disease, condition, or injury.
The CDMRP began in 1992 when the breast cancer advocacy community launched a grassroots effort to raise public awareness of the need for increased federal funding for breast cancer research. These advocates requested from Congress additional research funding to support innovative, high-impact research where the government was willing to take a risk to leapfrog the field forward. In response, Congress added funds to the DoD budget for breast cancer research, and the Breast Cancer Research Program (BCRP) was established with a fiscal year (FY) 1992 congressional appropriation.
The CDMRP brought a flexible, efficient way of managing research and was enthusiastic about the advocates’ desire to have a voice in setting research priorities. Since the initial appropriation, advocates representing breast cancer, ovarian cancer, prostate cancer, neurofibromatosis, and a wide range of other diseases, conditions, and injuries have demonstrated the need to Congress to appropriate funds for their respective causes.
There are several features that differentiate the CDMRP from other funding agencies. The most significant differences follow:
- The CDMRP funds innovative high-risk/high-gain research focused on the disease, condition, or injury as specified in congressional language;
- Unlike other agencies, the CDMRP integrates patients, survivors, family members, or caregivers of a person living with the disease, condition, or injury into every aspect of the program management cycle; and
- Every year the CDMRP programs develop a new investment strategy and release award mechanisms based on the most critical needs and scientific gaps.
These features ensure that the research funded in each program is relevant and has a high potential for impact in the patient community.
Funding and Science Management
Funding for the programs managed by the CDMRP does not appear as part of the DoD core funding in the president’s budget; instead, Congress assesses the needs of its constituents and adds funding to the DoD budget, designated specifically to meet those needs on an annual basis. Management of the CDMRP is funded entirely out of the annual appropriation, and there is no financial burden to the DoD. Unlike other federally funded agencies that receive funding in the president’s budget every FY, each CDMRP program develops an investment strategy based on a single yearly congressional appropriation.
Full project funding is obligated at the start from the single FY appropriation, ensuring multiyear research projects are not at funding risk. This method is in contrast to other agencies, which fund projects in budget years and may fund only a percentage of previously committed levels or cut the length of time for funding, depending on varying budget year funding policies.
Each CDMRP research program is managed by a multidisciplinary team and includes an external advisory board composed of world-renowned expert scientists, clinicians, and survivors from the DoD, National Institutes of Health (NIH), Centers for Disease Control and Prevention, VA, as well as academia and industry. Each research program has a vision/mission that is focused on ending or curing that disease, condition, or injury, ameliorating its consequences, or having a major impact on the quality of life of its survivors. Establishing a vision is the first major milestone in program execution, which enables each program to develop its individual investment strategy.
When establishing the investment strategy, each program evaluates the funding landscape by comparing research portfolios and award mechanisms within the organization as well as with other federal and nonfederal agencies. For some of the CDMRP-managed programs, such as the Peer Reviewed Orthopaedic Research Program, the Spinal Cord Injury Research Program, and the Psychological Health/Traumatic Brain Injury Research Program, topic areas are aligned with the Defense Health Program (DHP) Defense Medical Research and Development Program (DMRDP). The appropriate DHP Joint Program Committee provides guidance on military-relevant research priorities and uses oversight of all core and congressional special interest research efforts across the DoD services to complement and leverage projects with CDMRP funding.
Establishment of each program’s vision and investment strategy leads to the development of Program Announcements (PAs), which describe the intent of each award mechanism in order to solicit research applications aimed at making a significant and nonincremental impact. The PAs for each program as well as links to application submission are made available on the CDMRP webpage (http://cdmrp.army.mil/funding/prgdefault.shtml).
Emphasized in CDMRP research opportunities are the specific needs of its advocacy communities. The CDMRP recognizes the value of firsthand experience with each of the targeted diseases, conditions, and injuries and has been a leader in integrating consumers (defined as a patient, survivor, family member, or caregiver of a person living with the disease, condition, or injury) into every aspect of a program’s execution. The value of consumer involvement is derived from each individual’s firsthand experience. This approach adds a perspective, passion, and sense of urgency, which ensures that the human dimension is incorporated in each program’s policy, investment strategy, and research focus. Consumers vote side by side with scientists and clinicians on advisory boards for each of the programs, and they have since the inception of the CDMRP.
Each research application must have an impact statement describing how the proposed research, if successful, will transform an aspect of the understanding, prevention, detection, and/or treatment of the respective program area; ie, have an impact on the consumer community. The impact of the proposed research is a critical determinant of the funding recommendation.
Each research program’s investment strategy and associated award mechanisms provide the framework and direction necessary to most effectively invest the congressional appropriation. Operationally, the CDMRP monitors for potentially similar approaches in research at many milestones in its science management model to ensure that the CDMRP-funded research is synergistic and harmonizing, not duplicative of other federal and nonfederal sources of funding.
At the time of proposal submission, a comprehensive list of current and pending funding support for the principal investigator (PI) and all key personnel must be submitted. During the review process, peer reviewers who have extensive knowledge of the subject consult the pending and existing support documentation to ensure the research is complementary to what is already being investigated in the field. This ensures that the proposals recommended for funding are synergistic and contribute to the substantiation of data relevant to clinical decisions. After a project has been recommended for funding, the CDMRP scientific officers (ie, scientific technical advisors) check all available sources to ensure that the project to be funded is complementary to ongoing research. Last, during the period of performance, details about funding applied for and/or new funding obtained is required in the annual technical progress reports. Through this science management model, CDMRP ensures that funded research is complementary and able to innovatively fill gaps in the biomedical research pipeline.
Biomedical Funding
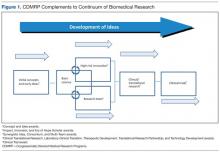
Most diseases, conditions, and injuries are complex, and finding a cure for them requires problem solving from multiple disciplines and approaches as well as validation of research results. Prior to the fielding and clinical application of knowledge and products, research spans a continuum from discovery to clinical trials. As shown in Figure 1, novel award mechanisms developed by the CDMRP programs facilitate the success of this research continuum and innovatively complement traditional research funding agencies, such as the NIH. The intent of each award mechanism is designed to solicit research proposals focused on the needs of the patient community and how they relate to the vision of the program.
The Research Continuum
Some CDMRP programs provide support along the entire continuum of research. Other programs, with less mature research fields, focus on funding more basic research. There are also CDMRP programs that place emphasis on clinical and advanced development research. Each program’s annual investment strategy and choice of award mechanisms is based on the needs of the patient and research communities, gaps in research, and other barriers to progress in curing, rehabilitating, or eliminating the disease, condition, or injury.
Fostering the Development of Ideas
Since its inception in 1992, the DoD BCRP has sought to fund innovative, ground-breaking research by encouraging “outside the box” thinking and fostering creative collaborations that have the potential to have a high impact toward the eradication of this disease. The BCRP has a proven history of developing novel award mechanisms to foster new approaches in research. For example, the Idea Award was developed in the initial years of the BCRP to support novel research with little or no preliminary data that could ultimately lead to a critical discovery or advancement in breast cancer research. At that time, such high-risk, but potentially high-reward research was determined to be significantly underfunded by existing agencies and was thus identified as a gap in funding. Several major advancements in breast cancer, including the development of trastuzumab, testing of sentinel lymph node biopsy, and discovery of BRCA2 and PTEN gene mutations, were supported in part with funding from the BCRP.
The Idea Award mechanism has been adopted by other CDMRP programs to introduce new paradigms, challenge current paradigms, or look at existing problems from new perspectives in other disease- or condition-focused research. To support the exploration of highly innovative, untested concepts or theories, the BCRP and the Prostate Cancer Research Program (PCRP) developed other award mechanisms known as the Concept Award and the Exploration-Hypothesis Development Award, respectively.
These award mechanisms supporting early concepts and ideas provided complementary and multiple approaches to the most traditional and well-known grant program: the NIH R01 (Research Project Grant Program). In general, an R01 award requires preliminary data, supports the next logical or incremental step, is knowledge focused, has no specific program requirements, and is not focused on a single disease or condition. One of the hallmarks of this type of early idea award was that the preliminary data could then be used to submit a research proposal to an NIH-like R01 award mechanism.
A recent survey of Idea Awards offered by the BCRP from 2006 to 2011 indicated that > 40% of awardees successfully obtained other sources of funding, more than half coming from the NIH. The NIH Common Fund, established in 2006, led to the creation of a high-risk/high-reward program with the Transformative Research Award, which is focused on innovation and challenging existing paradigms, unlike the R01 mechanism. This indicates that although other agencies have developed award mechanisms supporting pilot and feasibility studies (eg, R21 awards–Exploratory/Developmental Research Grant) and high-risk/high-reward research (eg, Transformative Research Award), CDMRP’s creation of these mechanisms has transformed biomedical research and remains an important vehicle in the idea development funding pipeline.
Facilitating Collaborative Partnerships
Many funding agencies have recognized that research collaborations are important for investigating the increasing complexity of disease, conditions, and injuries. The CDMRP-managed BCRP, Ovarian Cancer Research Program(OCRP), and PCRP created collaborative award mechanisms (eg, the Synergistic Idea Award) in which one research project is submitted by multiple investigators whose combined resources are leveraged and their expertise synergized to better address a research question. A unique aspect of these collaborative award mechanisms is that all the investigators (appropriately called partners) receive an individual award, not a subaward, incentivizing investigators to develop partnerships that might not otherwise be formed.
Rewarding Science Teams
Recognizing that research collaborations are important in investigating the increasing complexity of disease and injuries, several of the CDMRP research programs have developed team science award mechanisms. Using the Manhattan Project as a successful example of bringing together the most talented scientists to conduct research and development simultaneously to quickly solve a common problem, the CDMRP Neurofibromatosis Research Program developed a consortium award mechanism to establish consortia of exceptional investigators to conceive, develop, and conduct collaborative pilot, phase 1, and phase 2 clinical evaluations. To the authors’ knowledge, this is the largest dedicated effort in neurofibromatosis research to date. This mechanism has been adopted by several other CDMRP programs to focus on multidisciplinary approaches with investigators from multiple institutions, to address high-impact research ideas or unmet needs.
The PCRP used this framework to support the infrastructure necessary for a consortium consisting of 13 major U.S. cancer centers (Prostate Cancer Clinical Trials Consortium [PCCTC]) to rapidly execute early-phase clinical trials of therapeutic agents. The PCCTC consortium now conducts about 25% of all early-phase U.S. clinical trials for prostate cancer and has dramatically impacted the speed at which new options for therapy are available to patients. For example, the drug abiraterone acetate was brought through clinical testing in half the time typically required and represents a new option in the treatment of metastatic prostate cancer. In addition, the PCCTC also brought MDV3100, another therapy for advanced disease, rapidly through all phases of clinical testing.
The Lung Cancer Research Program (LCRP) used the consortium award mechanism to create a unique, early detection clinical consortium that includes 4 academic organizations, 4 military treatment facilities, and 7 VA facilities to focus on characterizing, developing, and/or improving early detection modalities for lung cancer. The BCRP has recently introduced the Multi-Team and Transformative Vision Award mechanisms to support innovative teams of scientists, clinicians, and breast cancer survivors, patients, family members, and persons affected by and/or at risk of breast cancer to work together toward making breakthroughs that may have a revolutionary impact in breast cancer prevention or treatment.
Collectively, these team science mechanisms facilitate the exchange of ideas and bring together individuals with special knowledge and skills needed to sustain cross-fertilization. Such collaborations can unravel complex phenomena and significantly accelerate progress, thus shrinking the pipeline of traditional reductionist approaches to novel discoveries and outcomes.
Encouraging Visionary Individuals
The BCRP has developed a series of award mechanisms that seek to identify and fund individuals with potential for, or a history of, extraordinary innovation and creativity at varying career stages, from predoctoral training through established investigators. The BCRP Era of Hope Scholar (EOHS) Award supports early-career researchers who are the best and brightest in their field(s) and therefore have a high potential for innovation in breast cancer research.
While demonstrated experience in forming effective partnerships and collaborations is a requirement, experience in breast cancer is not, encouraging applicants to challenge current dogma and look beyond tradition and convention already established in the field. The unique intent of this mechanism changed the way innovative science is reviewed, since the individual young investigator, rather than the project, is the central feature of this award. The BCRP Innovator Award supports established, visionary individuals, who have demonstrated creativity, innovative work, and leadership in any field. This mechanism also broke new ground by providing individuals with the funding and freedom to pursue their most novel, visionary, high-risk ideas that could ultimately lead to ending disease.
Dr. Greg Hannon of Cold Spring Harbor Laboratory received a BCRP New Investigator Award in FY 1995 and was one of the first recipients of the Innovator Award in FY 2001, making scientific breakthroughs in understanding the mechanisms of RNA interference. He is currently applying these discoveries to the identification of new therapeutic targets for breast cancer. By funding such individuals at different stages of their research career, the BCRP has provided the foundation for many of today’s leading breast cancer researchers. Moreover, innovative researchers, such as Dr. Hannon, have moved from other fields into the breast cancer field as a result of BCRP funding.
Another investigator who transitioned into distinct disease fields as a result of CDMRP funding is. From 2002 to 2007, Dr. Chinnaiyan received funding from the PCRP and made a paradigm-shifting discovery and identified multiple recurrent gene fusions in human prostate cancers. Dr. Chinnaiyan had not worked in prostate cancer before embarking on his groundbreaking studies and is now a leader in that field. In 2007, Dr. Chinnaiyan had a vision that characterization of recurrent gene fusions within human breast cancers could lead to the identification of new biomarkers and therapeutic targets for this disease. He was awarded the BCRP EOHS Award and went on to make an exciting discovery of 2 novel recurrent and actionable gene fusions in breast cancer, the results of which were published in 2011.1
Within the CDMRP, the OCRP has adopted the Innovator Award mechanism to attract visionary individuals from any field of research to focus their creativity, innovation, and leadership on ovarian cancer research. Through the use of this mechanism, this program has been successful in funding several noncancer scientists, including engineers, to help solve biomedical problems in the field. Six years after the initial release by CDMRP, the NIH introduced the Director’s New Innovator Award and the Pioneer Award. This CDMRP novel mechanism seems to have transformed funding strategies by encouraging innovative individuals to provide solutions to the toughest medical challenges.
Translation of Science to the Clinic
A critical component in the research continuum is the translation of promising lead agents to clinical trials. The CDMRP programs uniquely address clinical/translational research by focusing on critical needs and specific gaps within a particular disease, condition, or injury rather than a broad investment in general translational research. For example, the PCRP Laboratory-Clinical Transition Award mechanism supports product-driven preclinical studies of promising lead agents or medical devices that have the potential to revolutionize prostate cancer clinical care. For this award mechanism, lead agent development projects generate preclinical data to be used for an FDA investigational device exemption application and/or current Good Manufacturing Practice production of a medical device.
Preclinical Awards
The CDMRP Amyotrophic Lateral Sclerosis (ALS) Research Program focuses on the preclinical development of new therapies using the Therapeutic Development Award mechanism, which is product-driven and supports preclinical assessment of therapeutics, and the Therapeutic Idea Award mechanism, which promotes new ideas for novel therapeutics. This preclinical focus on therapeutic development compliments that of the National Institute of Neurological Disorders and Stroke (NINDS), the major NIH funder of ALS research, which concentrates primarily on funding basic and clinical research.
The CDMRP Gulf War Illness (GWI) Research Program created a unique award mechanism, the Innovative Treatment Evaluation Award, to support the early systematic evaluation of innovative treatment interventions that can provide proof of principle data for broader efficacy trials. The only other major funder of GWI research is the VA Office of Research and Development, which relies on the individual research interests of its intramural investigators.
In support of the 2012 Presidential Executive Order, the DoD and VA devoted > $100 million to fund 2 new consortia aimed at improving diagnosis and treatment of mild traumatic brain injury and posttraumatic stress disorder (PTSD). The Consortium to Alleviate PTSD and the Chronic Effects of Neurotrauma Consortium are jointly managed by the VA and CDMRP on behalf of the DoD and bring together leading scientists and clinicians devoted to the health and welfare of our nation’s service members and veterans. Consortium efforts are expected to have an emphasis toward translational/clinical work.
The OCRP developed the Translational Research Partnership Award mechanism to move an observation from the laboratory into clinical application for ovarian cancer. The novelty of this award is that one partner in the collaboration is required to be a laboratory scientist and the other is required to be a clinician. This award mechanism has been adopted by several other CDMRP research programs; a comparable mechanism has not been offered by other funding agencies.
The Peer Reviewed Orthopaedic Research Program (PRORP) is the only major funding source dedicated to research in combat and combat-related orthopedic injuries, the largest source of long-term morbidity for injured military personnel. The PRORP crafts investment strategies to address these challenges using award mechanisms such as the Technology Development, Translational Partnership, and the Clinical Trial awards, which emphasize clinical and mature translational research. While industry, including pharmaceutical, biotechnology, and medical device firms, remains the largest funder of clinical trial research, the CDMRP’s niche in this arena is the ability to encourage preventive or therapeutic interventions that are in line with the priorities of the communities affected by the disease.
Training and Career Development
Training the next generation of scientists in both basic and clinical research is instrumental for the advancement of biomedical research. The career development pipeline traditionally proceeds from predoctoral through postdoctoral training to the new or junior faculty level investigator (Figure 2). The CDMRP offers pre- and postdoctoral training award mechanisms in which the research is disease- or condition-specific, and the trainee is named as the principal investigator, thereby providing the trainee with his or her first source of research funding.
The BCRP Postdoctoral Award mechanism is unique in that it provides funding for the research in addition to stipend support. The trainee is expected to have discretion over management of the award, thus providing valuable training as a researcher. In addition to recent doctoral graduates, many CDMRP training mechanisms support recent medical graduates and encourage the training of physician-scientists. For example, the Prostate Cancer Research Program (PCRP) supports the training of physicians with clinical duties for a career in prostate cancer research through the physician Research Training Award. At the time of application, the PI must be in the last year of medical residency, must designate a mentor with an established research program, and institutions must provide at least 40% protection of the PI’s time for research.
The PRORP offers a career development award in which active-duty military researchers, physical therapists, occupational therapists, or physician-scientists with < 8 years of clinical or postdoctoral research experience (excluding clinical residency or medical fellowship training) are eligible to apply. The LCRP has offered a promising clinician research award supporting the training of MDs or MD/PhDs with clinical duties and/or responsibilities that are within 5 years of a professional appointment. Each of these physician training mechanisms not only provide support at an early career stage to investigators at DoD or other clinical sites, but also enable physicians to have a career at the forefront of research and clinical practice.
In 2002, the NIH observed that the percentage of competing NIH grants awarded to investigators aged ≤ 35 years declined from 23% in 1980 to < 4% in 2002. To support these young investigators, the NIH introduced the Pathway to Independence Award mechanism, providing several years of mentored support for promising postdoctoral scientists followed by several years of independent support. More recently, the NIH has focused on new investigators by offering more R01 awards to this group. Because securing early concept funding helps pave the way for the larger R01 grant application, increasing the support for early investigators is important for creating a critical threshold of scientists with exceptional talent.
The CDMRP New Investigator Award programs are intended to support scientists in the early stages of their careers through the continued development of promising independent investigators and/or the transition of established investigators. Each of the CDMRP programs has developed variations of the New Investigator Award to meet the goals of the disease- or condition-specific program. The OCRP Ovarian Cancer Academy, which includes both medical doctors and PhD scientists, is one of the more recent and innovative New Investigator Award mechanisms. This academy is an interactive virtual research and training platform that provides intensive mentoring, national networking, and a peer group for junior faculty in a collaborative and interactive environment. Taken together, the CDMRP programs offer unique award mechanisms to support researchers at critical junctures in their careers. The unique qualities and competitiveness of the CDMRP’s disease/condition-focused training awards have supported the early-career foundation for many of today’s leading researchers.
Conclusions
Congressionally Directed Medical Research Programs complement other federal and nonfederal sources of biomedical research funding and fill important research gaps through an evaluation of the funding landscape, identification of research gaps, and development of novel award mechanisms. The integration of survivors, patients, and their family members ensures that every aspect of the program management cycle balances scientific expertise with human perspective and has high impact on the patient community.
As new needs emerge, each research program designs an investment strategy to target areas most critically in need. The subsequent release of novel award mechanisms focuses research and enables an acceleration of science and/or leading researchers to the patient’s bedside. The CDMRP-funded discoveries have contributed to the development of new therapeutics, new diagnostics, and to changes in the standard of care exemplifying significant clinical impact and the innovative nature of these Congressional Special Interest Medical Research Programs.
To learn more about CDMRP or to receive funding notifications by e-mail, please visit http://cdmrp.army.mil.
Acknowledgments
The authors gratefully note the CDMRP Program Evaluation Steering Committee for critical review of the manuscript. Also acknowledged are CDMRP staff at large and Dr. Lisa Kinnard for support in this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Robinson DR, Kalyana-Sundaram S, Wu Y-I, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature Med. 2011;17(12):1646-1651.
The Congressionally Directed Medical Research Programs (CDMRP), an office within the U.S. Army Medical Research and Materiel Command, has executed funding for research in 18 biomedical programs (Table). These programs touch the lives of service members, veterans, family members, and the general public. A partnership with the military, government, scientific community, survivors, patients, and their family members brings together spheres of stakeholders that typically might not otherwise collaborate and enables the CDMRP to complement other sources of research funding while focusing on research most directly relevant to each disease, condition, or injury.
The CDMRP began in 1992 when the breast cancer advocacy community launched a grassroots effort to raise public awareness of the need for increased federal funding for breast cancer research. These advocates requested from Congress additional research funding to support innovative, high-impact research where the government was willing to take a risk to leapfrog the field forward. In response, Congress added funds to the DoD budget for breast cancer research, and the Breast Cancer Research Program (BCRP) was established with a fiscal year (FY) 1992 congressional appropriation.
The CDMRP brought a flexible, efficient way of managing research and was enthusiastic about the advocates’ desire to have a voice in setting research priorities. Since the initial appropriation, advocates representing breast cancer, ovarian cancer, prostate cancer, neurofibromatosis, and a wide range of other diseases, conditions, and injuries have demonstrated the need to Congress to appropriate funds for their respective causes.
There are several features that differentiate the CDMRP from other funding agencies. The most significant differences follow:
- The CDMRP funds innovative high-risk/high-gain research focused on the disease, condition, or injury as specified in congressional language;
- Unlike other agencies, the CDMRP integrates patients, survivors, family members, or caregivers of a person living with the disease, condition, or injury into every aspect of the program management cycle; and
- Every year the CDMRP programs develop a new investment strategy and release award mechanisms based on the most critical needs and scientific gaps.
These features ensure that the research funded in each program is relevant and has a high potential for impact in the patient community.
Funding and Science Management
Funding for the programs managed by the CDMRP does not appear as part of the DoD core funding in the president’s budget; instead, Congress assesses the needs of its constituents and adds funding to the DoD budget, designated specifically to meet those needs on an annual basis. Management of the CDMRP is funded entirely out of the annual appropriation, and there is no financial burden to the DoD. Unlike other federally funded agencies that receive funding in the president’s budget every FY, each CDMRP program develops an investment strategy based on a single yearly congressional appropriation.
Full project funding is obligated at the start from the single FY appropriation, ensuring multiyear research projects are not at funding risk. This method is in contrast to other agencies, which fund projects in budget years and may fund only a percentage of previously committed levels or cut the length of time for funding, depending on varying budget year funding policies.
Each CDMRP research program is managed by a multidisciplinary team and includes an external advisory board composed of world-renowned expert scientists, clinicians, and survivors from the DoD, National Institutes of Health (NIH), Centers for Disease Control and Prevention, VA, as well as academia and industry. Each research program has a vision/mission that is focused on ending or curing that disease, condition, or injury, ameliorating its consequences, or having a major impact on the quality of life of its survivors. Establishing a vision is the first major milestone in program execution, which enables each program to develop its individual investment strategy.
When establishing the investment strategy, each program evaluates the funding landscape by comparing research portfolios and award mechanisms within the organization as well as with other federal and nonfederal agencies. For some of the CDMRP-managed programs, such as the Peer Reviewed Orthopaedic Research Program, the Spinal Cord Injury Research Program, and the Psychological Health/Traumatic Brain Injury Research Program, topic areas are aligned with the Defense Health Program (DHP) Defense Medical Research and Development Program (DMRDP). The appropriate DHP Joint Program Committee provides guidance on military-relevant research priorities and uses oversight of all core and congressional special interest research efforts across the DoD services to complement and leverage projects with CDMRP funding.
Establishment of each program’s vision and investment strategy leads to the development of Program Announcements (PAs), which describe the intent of each award mechanism in order to solicit research applications aimed at making a significant and nonincremental impact. The PAs for each program as well as links to application submission are made available on the CDMRP webpage (http://cdmrp.army.mil/funding/prgdefault.shtml).
Emphasized in CDMRP research opportunities are the specific needs of its advocacy communities. The CDMRP recognizes the value of firsthand experience with each of the targeted diseases, conditions, and injuries and has been a leader in integrating consumers (defined as a patient, survivor, family member, or caregiver of a person living with the disease, condition, or injury) into every aspect of a program’s execution. The value of consumer involvement is derived from each individual’s firsthand experience. This approach adds a perspective, passion, and sense of urgency, which ensures that the human dimension is incorporated in each program’s policy, investment strategy, and research focus. Consumers vote side by side with scientists and clinicians on advisory boards for each of the programs, and they have since the inception of the CDMRP.
Each research application must have an impact statement describing how the proposed research, if successful, will transform an aspect of the understanding, prevention, detection, and/or treatment of the respective program area; ie, have an impact on the consumer community. The impact of the proposed research is a critical determinant of the funding recommendation.
Each research program’s investment strategy and associated award mechanisms provide the framework and direction necessary to most effectively invest the congressional appropriation. Operationally, the CDMRP monitors for potentially similar approaches in research at many milestones in its science management model to ensure that the CDMRP-funded research is synergistic and harmonizing, not duplicative of other federal and nonfederal sources of funding.
At the time of proposal submission, a comprehensive list of current and pending funding support for the principal investigator (PI) and all key personnel must be submitted. During the review process, peer reviewers who have extensive knowledge of the subject consult the pending and existing support documentation to ensure the research is complementary to what is already being investigated in the field. This ensures that the proposals recommended for funding are synergistic and contribute to the substantiation of data relevant to clinical decisions. After a project has been recommended for funding, the CDMRP scientific officers (ie, scientific technical advisors) check all available sources to ensure that the project to be funded is complementary to ongoing research. Last, during the period of performance, details about funding applied for and/or new funding obtained is required in the annual technical progress reports. Through this science management model, CDMRP ensures that funded research is complementary and able to innovatively fill gaps in the biomedical research pipeline.
Biomedical Funding
Most diseases, conditions, and injuries are complex, and finding a cure for them requires problem solving from multiple disciplines and approaches as well as validation of research results. Prior to the fielding and clinical application of knowledge and products, research spans a continuum from discovery to clinical trials. As shown in Figure 1, novel award mechanisms developed by the CDMRP programs facilitate the success of this research continuum and innovatively complement traditional research funding agencies, such as the NIH. The intent of each award mechanism is designed to solicit research proposals focused on the needs of the patient community and how they relate to the vision of the program.
The Research Continuum
Some CDMRP programs provide support along the entire continuum of research. Other programs, with less mature research fields, focus on funding more basic research. There are also CDMRP programs that place emphasis on clinical and advanced development research. Each program’s annual investment strategy and choice of award mechanisms is based on the needs of the patient and research communities, gaps in research, and other barriers to progress in curing, rehabilitating, or eliminating the disease, condition, or injury.
Fostering the Development of Ideas
Since its inception in 1992, the DoD BCRP has sought to fund innovative, ground-breaking research by encouraging “outside the box” thinking and fostering creative collaborations that have the potential to have a high impact toward the eradication of this disease. The BCRP has a proven history of developing novel award mechanisms to foster new approaches in research. For example, the Idea Award was developed in the initial years of the BCRP to support novel research with little or no preliminary data that could ultimately lead to a critical discovery or advancement in breast cancer research. At that time, such high-risk, but potentially high-reward research was determined to be significantly underfunded by existing agencies and was thus identified as a gap in funding. Several major advancements in breast cancer, including the development of trastuzumab, testing of sentinel lymph node biopsy, and discovery of BRCA2 and PTEN gene mutations, were supported in part with funding from the BCRP.
The Idea Award mechanism has been adopted by other CDMRP programs to introduce new paradigms, challenge current paradigms, or look at existing problems from new perspectives in other disease- or condition-focused research. To support the exploration of highly innovative, untested concepts or theories, the BCRP and the Prostate Cancer Research Program (PCRP) developed other award mechanisms known as the Concept Award and the Exploration-Hypothesis Development Award, respectively.
These award mechanisms supporting early concepts and ideas provided complementary and multiple approaches to the most traditional and well-known grant program: the NIH R01 (Research Project Grant Program). In general, an R01 award requires preliminary data, supports the next logical or incremental step, is knowledge focused, has no specific program requirements, and is not focused on a single disease or condition. One of the hallmarks of this type of early idea award was that the preliminary data could then be used to submit a research proposal to an NIH-like R01 award mechanism.
A recent survey of Idea Awards offered by the BCRP from 2006 to 2011 indicated that > 40% of awardees successfully obtained other sources of funding, more than half coming from the NIH. The NIH Common Fund, established in 2006, led to the creation of a high-risk/high-reward program with the Transformative Research Award, which is focused on innovation and challenging existing paradigms, unlike the R01 mechanism. This indicates that although other agencies have developed award mechanisms supporting pilot and feasibility studies (eg, R21 awards–Exploratory/Developmental Research Grant) and high-risk/high-reward research (eg, Transformative Research Award), CDMRP’s creation of these mechanisms has transformed biomedical research and remains an important vehicle in the idea development funding pipeline.
Facilitating Collaborative Partnerships
Many funding agencies have recognized that research collaborations are important for investigating the increasing complexity of disease, conditions, and injuries. The CDMRP-managed BCRP, Ovarian Cancer Research Program(OCRP), and PCRP created collaborative award mechanisms (eg, the Synergistic Idea Award) in which one research project is submitted by multiple investigators whose combined resources are leveraged and their expertise synergized to better address a research question. A unique aspect of these collaborative award mechanisms is that all the investigators (appropriately called partners) receive an individual award, not a subaward, incentivizing investigators to develop partnerships that might not otherwise be formed.
Rewarding Science Teams
Recognizing that research collaborations are important in investigating the increasing complexity of disease and injuries, several of the CDMRP research programs have developed team science award mechanisms. Using the Manhattan Project as a successful example of bringing together the most talented scientists to conduct research and development simultaneously to quickly solve a common problem, the CDMRP Neurofibromatosis Research Program developed a consortium award mechanism to establish consortia of exceptional investigators to conceive, develop, and conduct collaborative pilot, phase 1, and phase 2 clinical evaluations. To the authors’ knowledge, this is the largest dedicated effort in neurofibromatosis research to date. This mechanism has been adopted by several other CDMRP programs to focus on multidisciplinary approaches with investigators from multiple institutions, to address high-impact research ideas or unmet needs.
The PCRP used this framework to support the infrastructure necessary for a consortium consisting of 13 major U.S. cancer centers (Prostate Cancer Clinical Trials Consortium [PCCTC]) to rapidly execute early-phase clinical trials of therapeutic agents. The PCCTC consortium now conducts about 25% of all early-phase U.S. clinical trials for prostate cancer and has dramatically impacted the speed at which new options for therapy are available to patients. For example, the drug abiraterone acetate was brought through clinical testing in half the time typically required and represents a new option in the treatment of metastatic prostate cancer. In addition, the PCCTC also brought MDV3100, another therapy for advanced disease, rapidly through all phases of clinical testing.
The Lung Cancer Research Program (LCRP) used the consortium award mechanism to create a unique, early detection clinical consortium that includes 4 academic organizations, 4 military treatment facilities, and 7 VA facilities to focus on characterizing, developing, and/or improving early detection modalities for lung cancer. The BCRP has recently introduced the Multi-Team and Transformative Vision Award mechanisms to support innovative teams of scientists, clinicians, and breast cancer survivors, patients, family members, and persons affected by and/or at risk of breast cancer to work together toward making breakthroughs that may have a revolutionary impact in breast cancer prevention or treatment.
Collectively, these team science mechanisms facilitate the exchange of ideas and bring together individuals with special knowledge and skills needed to sustain cross-fertilization. Such collaborations can unravel complex phenomena and significantly accelerate progress, thus shrinking the pipeline of traditional reductionist approaches to novel discoveries and outcomes.
Encouraging Visionary Individuals
The BCRP has developed a series of award mechanisms that seek to identify and fund individuals with potential for, or a history of, extraordinary innovation and creativity at varying career stages, from predoctoral training through established investigators. The BCRP Era of Hope Scholar (EOHS) Award supports early-career researchers who are the best and brightest in their field(s) and therefore have a high potential for innovation in breast cancer research.
While demonstrated experience in forming effective partnerships and collaborations is a requirement, experience in breast cancer is not, encouraging applicants to challenge current dogma and look beyond tradition and convention already established in the field. The unique intent of this mechanism changed the way innovative science is reviewed, since the individual young investigator, rather than the project, is the central feature of this award. The BCRP Innovator Award supports established, visionary individuals, who have demonstrated creativity, innovative work, and leadership in any field. This mechanism also broke new ground by providing individuals with the funding and freedom to pursue their most novel, visionary, high-risk ideas that could ultimately lead to ending disease.
Dr. Greg Hannon of Cold Spring Harbor Laboratory received a BCRP New Investigator Award in FY 1995 and was one of the first recipients of the Innovator Award in FY 2001, making scientific breakthroughs in understanding the mechanisms of RNA interference. He is currently applying these discoveries to the identification of new therapeutic targets for breast cancer. By funding such individuals at different stages of their research career, the BCRP has provided the foundation for many of today’s leading breast cancer researchers. Moreover, innovative researchers, such as Dr. Hannon, have moved from other fields into the breast cancer field as a result of BCRP funding.
Another investigator who transitioned into distinct disease fields as a result of CDMRP funding is. From 2002 to 2007, Dr. Chinnaiyan received funding from the PCRP and made a paradigm-shifting discovery and identified multiple recurrent gene fusions in human prostate cancers. Dr. Chinnaiyan had not worked in prostate cancer before embarking on his groundbreaking studies and is now a leader in that field. In 2007, Dr. Chinnaiyan had a vision that characterization of recurrent gene fusions within human breast cancers could lead to the identification of new biomarkers and therapeutic targets for this disease. He was awarded the BCRP EOHS Award and went on to make an exciting discovery of 2 novel recurrent and actionable gene fusions in breast cancer, the results of which were published in 2011.1
Within the CDMRP, the OCRP has adopted the Innovator Award mechanism to attract visionary individuals from any field of research to focus their creativity, innovation, and leadership on ovarian cancer research. Through the use of this mechanism, this program has been successful in funding several noncancer scientists, including engineers, to help solve biomedical problems in the field. Six years after the initial release by CDMRP, the NIH introduced the Director’s New Innovator Award and the Pioneer Award. This CDMRP novel mechanism seems to have transformed funding strategies by encouraging innovative individuals to provide solutions to the toughest medical challenges.
Translation of Science to the Clinic
A critical component in the research continuum is the translation of promising lead agents to clinical trials. The CDMRP programs uniquely address clinical/translational research by focusing on critical needs and specific gaps within a particular disease, condition, or injury rather than a broad investment in general translational research. For example, the PCRP Laboratory-Clinical Transition Award mechanism supports product-driven preclinical studies of promising lead agents or medical devices that have the potential to revolutionize prostate cancer clinical care. For this award mechanism, lead agent development projects generate preclinical data to be used for an FDA investigational device exemption application and/or current Good Manufacturing Practice production of a medical device.
Preclinical Awards
The CDMRP Amyotrophic Lateral Sclerosis (ALS) Research Program focuses on the preclinical development of new therapies using the Therapeutic Development Award mechanism, which is product-driven and supports preclinical assessment of therapeutics, and the Therapeutic Idea Award mechanism, which promotes new ideas for novel therapeutics. This preclinical focus on therapeutic development compliments that of the National Institute of Neurological Disorders and Stroke (NINDS), the major NIH funder of ALS research, which concentrates primarily on funding basic and clinical research.
The CDMRP Gulf War Illness (GWI) Research Program created a unique award mechanism, the Innovative Treatment Evaluation Award, to support the early systematic evaluation of innovative treatment interventions that can provide proof of principle data for broader efficacy trials. The only other major funder of GWI research is the VA Office of Research and Development, which relies on the individual research interests of its intramural investigators.
In support of the 2012 Presidential Executive Order, the DoD and VA devoted > $100 million to fund 2 new consortia aimed at improving diagnosis and treatment of mild traumatic brain injury and posttraumatic stress disorder (PTSD). The Consortium to Alleviate PTSD and the Chronic Effects of Neurotrauma Consortium are jointly managed by the VA and CDMRP on behalf of the DoD and bring together leading scientists and clinicians devoted to the health and welfare of our nation’s service members and veterans. Consortium efforts are expected to have an emphasis toward translational/clinical work.
The OCRP developed the Translational Research Partnership Award mechanism to move an observation from the laboratory into clinical application for ovarian cancer. The novelty of this award is that one partner in the collaboration is required to be a laboratory scientist and the other is required to be a clinician. This award mechanism has been adopted by several other CDMRP research programs; a comparable mechanism has not been offered by other funding agencies.
The Peer Reviewed Orthopaedic Research Program (PRORP) is the only major funding source dedicated to research in combat and combat-related orthopedic injuries, the largest source of long-term morbidity for injured military personnel. The PRORP crafts investment strategies to address these challenges using award mechanisms such as the Technology Development, Translational Partnership, and the Clinical Trial awards, which emphasize clinical and mature translational research. While industry, including pharmaceutical, biotechnology, and medical device firms, remains the largest funder of clinical trial research, the CDMRP’s niche in this arena is the ability to encourage preventive or therapeutic interventions that are in line with the priorities of the communities affected by the disease.
Training and Career Development
Training the next generation of scientists in both basic and clinical research is instrumental for the advancement of biomedical research. The career development pipeline traditionally proceeds from predoctoral through postdoctoral training to the new or junior faculty level investigator (Figure 2). The CDMRP offers pre- and postdoctoral training award mechanisms in which the research is disease- or condition-specific, and the trainee is named as the principal investigator, thereby providing the trainee with his or her first source of research funding.
The BCRP Postdoctoral Award mechanism is unique in that it provides funding for the research in addition to stipend support. The trainee is expected to have discretion over management of the award, thus providing valuable training as a researcher. In addition to recent doctoral graduates, many CDMRP training mechanisms support recent medical graduates and encourage the training of physician-scientists. For example, the Prostate Cancer Research Program (PCRP) supports the training of physicians with clinical duties for a career in prostate cancer research through the physician Research Training Award. At the time of application, the PI must be in the last year of medical residency, must designate a mentor with an established research program, and institutions must provide at least 40% protection of the PI’s time for research.
The PRORP offers a career development award in which active-duty military researchers, physical therapists, occupational therapists, or physician-scientists with < 8 years of clinical or postdoctoral research experience (excluding clinical residency or medical fellowship training) are eligible to apply. The LCRP has offered a promising clinician research award supporting the training of MDs or MD/PhDs with clinical duties and/or responsibilities that are within 5 years of a professional appointment. Each of these physician training mechanisms not only provide support at an early career stage to investigators at DoD or other clinical sites, but also enable physicians to have a career at the forefront of research and clinical practice.
In 2002, the NIH observed that the percentage of competing NIH grants awarded to investigators aged ≤ 35 years declined from 23% in 1980 to < 4% in 2002. To support these young investigators, the NIH introduced the Pathway to Independence Award mechanism, providing several years of mentored support for promising postdoctoral scientists followed by several years of independent support. More recently, the NIH has focused on new investigators by offering more R01 awards to this group. Because securing early concept funding helps pave the way for the larger R01 grant application, increasing the support for early investigators is important for creating a critical threshold of scientists with exceptional talent.
The CDMRP New Investigator Award programs are intended to support scientists in the early stages of their careers through the continued development of promising independent investigators and/or the transition of established investigators. Each of the CDMRP programs has developed variations of the New Investigator Award to meet the goals of the disease- or condition-specific program. The OCRP Ovarian Cancer Academy, which includes both medical doctors and PhD scientists, is one of the more recent and innovative New Investigator Award mechanisms. This academy is an interactive virtual research and training platform that provides intensive mentoring, national networking, and a peer group for junior faculty in a collaborative and interactive environment. Taken together, the CDMRP programs offer unique award mechanisms to support researchers at critical junctures in their careers. The unique qualities and competitiveness of the CDMRP’s disease/condition-focused training awards have supported the early-career foundation for many of today’s leading researchers.
Conclusions
Congressionally Directed Medical Research Programs complement other federal and nonfederal sources of biomedical research funding and fill important research gaps through an evaluation of the funding landscape, identification of research gaps, and development of novel award mechanisms. The integration of survivors, patients, and their family members ensures that every aspect of the program management cycle balances scientific expertise with human perspective and has high impact on the patient community.
As new needs emerge, each research program designs an investment strategy to target areas most critically in need. The subsequent release of novel award mechanisms focuses research and enables an acceleration of science and/or leading researchers to the patient’s bedside. The CDMRP-funded discoveries have contributed to the development of new therapeutics, new diagnostics, and to changes in the standard of care exemplifying significant clinical impact and the innovative nature of these Congressional Special Interest Medical Research Programs.
To learn more about CDMRP or to receive funding notifications by e-mail, please visit http://cdmrp.army.mil.
Acknowledgments
The authors gratefully note the CDMRP Program Evaluation Steering Committee for critical review of the manuscript. Also acknowledged are CDMRP staff at large and Dr. Lisa Kinnard for support in this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The Congressionally Directed Medical Research Programs (CDMRP), an office within the U.S. Army Medical Research and Materiel Command, has executed funding for research in 18 biomedical programs (Table). These programs touch the lives of service members, veterans, family members, and the general public. A partnership with the military, government, scientific community, survivors, patients, and their family members brings together spheres of stakeholders that typically might not otherwise collaborate and enables the CDMRP to complement other sources of research funding while focusing on research most directly relevant to each disease, condition, or injury.
The CDMRP began in 1992 when the breast cancer advocacy community launched a grassroots effort to raise public awareness of the need for increased federal funding for breast cancer research. These advocates requested from Congress additional research funding to support innovative, high-impact research where the government was willing to take a risk to leapfrog the field forward. In response, Congress added funds to the DoD budget for breast cancer research, and the Breast Cancer Research Program (BCRP) was established with a fiscal year (FY) 1992 congressional appropriation.
The CDMRP brought a flexible, efficient way of managing research and was enthusiastic about the advocates’ desire to have a voice in setting research priorities. Since the initial appropriation, advocates representing breast cancer, ovarian cancer, prostate cancer, neurofibromatosis, and a wide range of other diseases, conditions, and injuries have demonstrated the need to Congress to appropriate funds for their respective causes.
There are several features that differentiate the CDMRP from other funding agencies. The most significant differences follow:
- The CDMRP funds innovative high-risk/high-gain research focused on the disease, condition, or injury as specified in congressional language;
- Unlike other agencies, the CDMRP integrates patients, survivors, family members, or caregivers of a person living with the disease, condition, or injury into every aspect of the program management cycle; and
- Every year the CDMRP programs develop a new investment strategy and release award mechanisms based on the most critical needs and scientific gaps.
These features ensure that the research funded in each program is relevant and has a high potential for impact in the patient community.
Funding and Science Management
Funding for the programs managed by the CDMRP does not appear as part of the DoD core funding in the president’s budget; instead, Congress assesses the needs of its constituents and adds funding to the DoD budget, designated specifically to meet those needs on an annual basis. Management of the CDMRP is funded entirely out of the annual appropriation, and there is no financial burden to the DoD. Unlike other federally funded agencies that receive funding in the president’s budget every FY, each CDMRP program develops an investment strategy based on a single yearly congressional appropriation.
Full project funding is obligated at the start from the single FY appropriation, ensuring multiyear research projects are not at funding risk. This method is in contrast to other agencies, which fund projects in budget years and may fund only a percentage of previously committed levels or cut the length of time for funding, depending on varying budget year funding policies.
Each CDMRP research program is managed by a multidisciplinary team and includes an external advisory board composed of world-renowned expert scientists, clinicians, and survivors from the DoD, National Institutes of Health (NIH), Centers for Disease Control and Prevention, VA, as well as academia and industry. Each research program has a vision/mission that is focused on ending or curing that disease, condition, or injury, ameliorating its consequences, or having a major impact on the quality of life of its survivors. Establishing a vision is the first major milestone in program execution, which enables each program to develop its individual investment strategy.
When establishing the investment strategy, each program evaluates the funding landscape by comparing research portfolios and award mechanisms within the organization as well as with other federal and nonfederal agencies. For some of the CDMRP-managed programs, such as the Peer Reviewed Orthopaedic Research Program, the Spinal Cord Injury Research Program, and the Psychological Health/Traumatic Brain Injury Research Program, topic areas are aligned with the Defense Health Program (DHP) Defense Medical Research and Development Program (DMRDP). The appropriate DHP Joint Program Committee provides guidance on military-relevant research priorities and uses oversight of all core and congressional special interest research efforts across the DoD services to complement and leverage projects with CDMRP funding.
Establishment of each program’s vision and investment strategy leads to the development of Program Announcements (PAs), which describe the intent of each award mechanism in order to solicit research applications aimed at making a significant and nonincremental impact. The PAs for each program as well as links to application submission are made available on the CDMRP webpage (http://cdmrp.army.mil/funding/prgdefault.shtml).
Emphasized in CDMRP research opportunities are the specific needs of its advocacy communities. The CDMRP recognizes the value of firsthand experience with each of the targeted diseases, conditions, and injuries and has been a leader in integrating consumers (defined as a patient, survivor, family member, or caregiver of a person living with the disease, condition, or injury) into every aspect of a program’s execution. The value of consumer involvement is derived from each individual’s firsthand experience. This approach adds a perspective, passion, and sense of urgency, which ensures that the human dimension is incorporated in each program’s policy, investment strategy, and research focus. Consumers vote side by side with scientists and clinicians on advisory boards for each of the programs, and they have since the inception of the CDMRP.
Each research application must have an impact statement describing how the proposed research, if successful, will transform an aspect of the understanding, prevention, detection, and/or treatment of the respective program area; ie, have an impact on the consumer community. The impact of the proposed research is a critical determinant of the funding recommendation.
Each research program’s investment strategy and associated award mechanisms provide the framework and direction necessary to most effectively invest the congressional appropriation. Operationally, the CDMRP monitors for potentially similar approaches in research at many milestones in its science management model to ensure that the CDMRP-funded research is synergistic and harmonizing, not duplicative of other federal and nonfederal sources of funding.
At the time of proposal submission, a comprehensive list of current and pending funding support for the principal investigator (PI) and all key personnel must be submitted. During the review process, peer reviewers who have extensive knowledge of the subject consult the pending and existing support documentation to ensure the research is complementary to what is already being investigated in the field. This ensures that the proposals recommended for funding are synergistic and contribute to the substantiation of data relevant to clinical decisions. After a project has been recommended for funding, the CDMRP scientific officers (ie, scientific technical advisors) check all available sources to ensure that the project to be funded is complementary to ongoing research. Last, during the period of performance, details about funding applied for and/or new funding obtained is required in the annual technical progress reports. Through this science management model, CDMRP ensures that funded research is complementary and able to innovatively fill gaps in the biomedical research pipeline.
Biomedical Funding
Most diseases, conditions, and injuries are complex, and finding a cure for them requires problem solving from multiple disciplines and approaches as well as validation of research results. Prior to the fielding and clinical application of knowledge and products, research spans a continuum from discovery to clinical trials. As shown in Figure 1, novel award mechanisms developed by the CDMRP programs facilitate the success of this research continuum and innovatively complement traditional research funding agencies, such as the NIH. The intent of each award mechanism is designed to solicit research proposals focused on the needs of the patient community and how they relate to the vision of the program.
The Research Continuum
Some CDMRP programs provide support along the entire continuum of research. Other programs, with less mature research fields, focus on funding more basic research. There are also CDMRP programs that place emphasis on clinical and advanced development research. Each program’s annual investment strategy and choice of award mechanisms is based on the needs of the patient and research communities, gaps in research, and other barriers to progress in curing, rehabilitating, or eliminating the disease, condition, or injury.
Fostering the Development of Ideas
Since its inception in 1992, the DoD BCRP has sought to fund innovative, ground-breaking research by encouraging “outside the box” thinking and fostering creative collaborations that have the potential to have a high impact toward the eradication of this disease. The BCRP has a proven history of developing novel award mechanisms to foster new approaches in research. For example, the Idea Award was developed in the initial years of the BCRP to support novel research with little or no preliminary data that could ultimately lead to a critical discovery or advancement in breast cancer research. At that time, such high-risk, but potentially high-reward research was determined to be significantly underfunded by existing agencies and was thus identified as a gap in funding. Several major advancements in breast cancer, including the development of trastuzumab, testing of sentinel lymph node biopsy, and discovery of BRCA2 and PTEN gene mutations, were supported in part with funding from the BCRP.
The Idea Award mechanism has been adopted by other CDMRP programs to introduce new paradigms, challenge current paradigms, or look at existing problems from new perspectives in other disease- or condition-focused research. To support the exploration of highly innovative, untested concepts or theories, the BCRP and the Prostate Cancer Research Program (PCRP) developed other award mechanisms known as the Concept Award and the Exploration-Hypothesis Development Award, respectively.
These award mechanisms supporting early concepts and ideas provided complementary and multiple approaches to the most traditional and well-known grant program: the NIH R01 (Research Project Grant Program). In general, an R01 award requires preliminary data, supports the next logical or incremental step, is knowledge focused, has no specific program requirements, and is not focused on a single disease or condition. One of the hallmarks of this type of early idea award was that the preliminary data could then be used to submit a research proposal to an NIH-like R01 award mechanism.
A recent survey of Idea Awards offered by the BCRP from 2006 to 2011 indicated that > 40% of awardees successfully obtained other sources of funding, more than half coming from the NIH. The NIH Common Fund, established in 2006, led to the creation of a high-risk/high-reward program with the Transformative Research Award, which is focused on innovation and challenging existing paradigms, unlike the R01 mechanism. This indicates that although other agencies have developed award mechanisms supporting pilot and feasibility studies (eg, R21 awards–Exploratory/Developmental Research Grant) and high-risk/high-reward research (eg, Transformative Research Award), CDMRP’s creation of these mechanisms has transformed biomedical research and remains an important vehicle in the idea development funding pipeline.
Facilitating Collaborative Partnerships
Many funding agencies have recognized that research collaborations are important for investigating the increasing complexity of disease, conditions, and injuries. The CDMRP-managed BCRP, Ovarian Cancer Research Program(OCRP), and PCRP created collaborative award mechanisms (eg, the Synergistic Idea Award) in which one research project is submitted by multiple investigators whose combined resources are leveraged and their expertise synergized to better address a research question. A unique aspect of these collaborative award mechanisms is that all the investigators (appropriately called partners) receive an individual award, not a subaward, incentivizing investigators to develop partnerships that might not otherwise be formed.
Rewarding Science Teams
Recognizing that research collaborations are important in investigating the increasing complexity of disease and injuries, several of the CDMRP research programs have developed team science award mechanisms. Using the Manhattan Project as a successful example of bringing together the most talented scientists to conduct research and development simultaneously to quickly solve a common problem, the CDMRP Neurofibromatosis Research Program developed a consortium award mechanism to establish consortia of exceptional investigators to conceive, develop, and conduct collaborative pilot, phase 1, and phase 2 clinical evaluations. To the authors’ knowledge, this is the largest dedicated effort in neurofibromatosis research to date. This mechanism has been adopted by several other CDMRP programs to focus on multidisciplinary approaches with investigators from multiple institutions, to address high-impact research ideas or unmet needs.
The PCRP used this framework to support the infrastructure necessary for a consortium consisting of 13 major U.S. cancer centers (Prostate Cancer Clinical Trials Consortium [PCCTC]) to rapidly execute early-phase clinical trials of therapeutic agents. The PCCTC consortium now conducts about 25% of all early-phase U.S. clinical trials for prostate cancer and has dramatically impacted the speed at which new options for therapy are available to patients. For example, the drug abiraterone acetate was brought through clinical testing in half the time typically required and represents a new option in the treatment of metastatic prostate cancer. In addition, the PCCTC also brought MDV3100, another therapy for advanced disease, rapidly through all phases of clinical testing.
The Lung Cancer Research Program (LCRP) used the consortium award mechanism to create a unique, early detection clinical consortium that includes 4 academic organizations, 4 military treatment facilities, and 7 VA facilities to focus on characterizing, developing, and/or improving early detection modalities for lung cancer. The BCRP has recently introduced the Multi-Team and Transformative Vision Award mechanisms to support innovative teams of scientists, clinicians, and breast cancer survivors, patients, family members, and persons affected by and/or at risk of breast cancer to work together toward making breakthroughs that may have a revolutionary impact in breast cancer prevention or treatment.
Collectively, these team science mechanisms facilitate the exchange of ideas and bring together individuals with special knowledge and skills needed to sustain cross-fertilization. Such collaborations can unravel complex phenomena and significantly accelerate progress, thus shrinking the pipeline of traditional reductionist approaches to novel discoveries and outcomes.
Encouraging Visionary Individuals
The BCRP has developed a series of award mechanisms that seek to identify and fund individuals with potential for, or a history of, extraordinary innovation and creativity at varying career stages, from predoctoral training through established investigators. The BCRP Era of Hope Scholar (EOHS) Award supports early-career researchers who are the best and brightest in their field(s) and therefore have a high potential for innovation in breast cancer research.
While demonstrated experience in forming effective partnerships and collaborations is a requirement, experience in breast cancer is not, encouraging applicants to challenge current dogma and look beyond tradition and convention already established in the field. The unique intent of this mechanism changed the way innovative science is reviewed, since the individual young investigator, rather than the project, is the central feature of this award. The BCRP Innovator Award supports established, visionary individuals, who have demonstrated creativity, innovative work, and leadership in any field. This mechanism also broke new ground by providing individuals with the funding and freedom to pursue their most novel, visionary, high-risk ideas that could ultimately lead to ending disease.
Dr. Greg Hannon of Cold Spring Harbor Laboratory received a BCRP New Investigator Award in FY 1995 and was one of the first recipients of the Innovator Award in FY 2001, making scientific breakthroughs in understanding the mechanisms of RNA interference. He is currently applying these discoveries to the identification of new therapeutic targets for breast cancer. By funding such individuals at different stages of their research career, the BCRP has provided the foundation for many of today’s leading breast cancer researchers. Moreover, innovative researchers, such as Dr. Hannon, have moved from other fields into the breast cancer field as a result of BCRP funding.
Another investigator who transitioned into distinct disease fields as a result of CDMRP funding is. From 2002 to 2007, Dr. Chinnaiyan received funding from the PCRP and made a paradigm-shifting discovery and identified multiple recurrent gene fusions in human prostate cancers. Dr. Chinnaiyan had not worked in prostate cancer before embarking on his groundbreaking studies and is now a leader in that field. In 2007, Dr. Chinnaiyan had a vision that characterization of recurrent gene fusions within human breast cancers could lead to the identification of new biomarkers and therapeutic targets for this disease. He was awarded the BCRP EOHS Award and went on to make an exciting discovery of 2 novel recurrent and actionable gene fusions in breast cancer, the results of which were published in 2011.1
Within the CDMRP, the OCRP has adopted the Innovator Award mechanism to attract visionary individuals from any field of research to focus their creativity, innovation, and leadership on ovarian cancer research. Through the use of this mechanism, this program has been successful in funding several noncancer scientists, including engineers, to help solve biomedical problems in the field. Six years after the initial release by CDMRP, the NIH introduced the Director’s New Innovator Award and the Pioneer Award. This CDMRP novel mechanism seems to have transformed funding strategies by encouraging innovative individuals to provide solutions to the toughest medical challenges.
Translation of Science to the Clinic
A critical component in the research continuum is the translation of promising lead agents to clinical trials. The CDMRP programs uniquely address clinical/translational research by focusing on critical needs and specific gaps within a particular disease, condition, or injury rather than a broad investment in general translational research. For example, the PCRP Laboratory-Clinical Transition Award mechanism supports product-driven preclinical studies of promising lead agents or medical devices that have the potential to revolutionize prostate cancer clinical care. For this award mechanism, lead agent development projects generate preclinical data to be used for an FDA investigational device exemption application and/or current Good Manufacturing Practice production of a medical device.
Preclinical Awards
The CDMRP Amyotrophic Lateral Sclerosis (ALS) Research Program focuses on the preclinical development of new therapies using the Therapeutic Development Award mechanism, which is product-driven and supports preclinical assessment of therapeutics, and the Therapeutic Idea Award mechanism, which promotes new ideas for novel therapeutics. This preclinical focus on therapeutic development compliments that of the National Institute of Neurological Disorders and Stroke (NINDS), the major NIH funder of ALS research, which concentrates primarily on funding basic and clinical research.
The CDMRP Gulf War Illness (GWI) Research Program created a unique award mechanism, the Innovative Treatment Evaluation Award, to support the early systematic evaluation of innovative treatment interventions that can provide proof of principle data for broader efficacy trials. The only other major funder of GWI research is the VA Office of Research and Development, which relies on the individual research interests of its intramural investigators.
In support of the 2012 Presidential Executive Order, the DoD and VA devoted > $100 million to fund 2 new consortia aimed at improving diagnosis and treatment of mild traumatic brain injury and posttraumatic stress disorder (PTSD). The Consortium to Alleviate PTSD and the Chronic Effects of Neurotrauma Consortium are jointly managed by the VA and CDMRP on behalf of the DoD and bring together leading scientists and clinicians devoted to the health and welfare of our nation’s service members and veterans. Consortium efforts are expected to have an emphasis toward translational/clinical work.
The OCRP developed the Translational Research Partnership Award mechanism to move an observation from the laboratory into clinical application for ovarian cancer. The novelty of this award is that one partner in the collaboration is required to be a laboratory scientist and the other is required to be a clinician. This award mechanism has been adopted by several other CDMRP research programs; a comparable mechanism has not been offered by other funding agencies.
The Peer Reviewed Orthopaedic Research Program (PRORP) is the only major funding source dedicated to research in combat and combat-related orthopedic injuries, the largest source of long-term morbidity for injured military personnel. The PRORP crafts investment strategies to address these challenges using award mechanisms such as the Technology Development, Translational Partnership, and the Clinical Trial awards, which emphasize clinical and mature translational research. While industry, including pharmaceutical, biotechnology, and medical device firms, remains the largest funder of clinical trial research, the CDMRP’s niche in this arena is the ability to encourage preventive or therapeutic interventions that are in line with the priorities of the communities affected by the disease.
Training and Career Development
Training the next generation of scientists in both basic and clinical research is instrumental for the advancement of biomedical research. The career development pipeline traditionally proceeds from predoctoral through postdoctoral training to the new or junior faculty level investigator (Figure 2). The CDMRP offers pre- and postdoctoral training award mechanisms in which the research is disease- or condition-specific, and the trainee is named as the principal investigator, thereby providing the trainee with his or her first source of research funding.
The BCRP Postdoctoral Award mechanism is unique in that it provides funding for the research in addition to stipend support. The trainee is expected to have discretion over management of the award, thus providing valuable training as a researcher. In addition to recent doctoral graduates, many CDMRP training mechanisms support recent medical graduates and encourage the training of physician-scientists. For example, the Prostate Cancer Research Program (PCRP) supports the training of physicians with clinical duties for a career in prostate cancer research through the physician Research Training Award. At the time of application, the PI must be in the last year of medical residency, must designate a mentor with an established research program, and institutions must provide at least 40% protection of the PI’s time for research.
The PRORP offers a career development award in which active-duty military researchers, physical therapists, occupational therapists, or physician-scientists with < 8 years of clinical or postdoctoral research experience (excluding clinical residency or medical fellowship training) are eligible to apply. The LCRP has offered a promising clinician research award supporting the training of MDs or MD/PhDs with clinical duties and/or responsibilities that are within 5 years of a professional appointment. Each of these physician training mechanisms not only provide support at an early career stage to investigators at DoD or other clinical sites, but also enable physicians to have a career at the forefront of research and clinical practice.
In 2002, the NIH observed that the percentage of competing NIH grants awarded to investigators aged ≤ 35 years declined from 23% in 1980 to < 4% in 2002. To support these young investigators, the NIH introduced the Pathway to Independence Award mechanism, providing several years of mentored support for promising postdoctoral scientists followed by several years of independent support. More recently, the NIH has focused on new investigators by offering more R01 awards to this group. Because securing early concept funding helps pave the way for the larger R01 grant application, increasing the support for early investigators is important for creating a critical threshold of scientists with exceptional talent.
The CDMRP New Investigator Award programs are intended to support scientists in the early stages of their careers through the continued development of promising independent investigators and/or the transition of established investigators. Each of the CDMRP programs has developed variations of the New Investigator Award to meet the goals of the disease- or condition-specific program. The OCRP Ovarian Cancer Academy, which includes both medical doctors and PhD scientists, is one of the more recent and innovative New Investigator Award mechanisms. This academy is an interactive virtual research and training platform that provides intensive mentoring, national networking, and a peer group for junior faculty in a collaborative and interactive environment. Taken together, the CDMRP programs offer unique award mechanisms to support researchers at critical junctures in their careers. The unique qualities and competitiveness of the CDMRP’s disease/condition-focused training awards have supported the early-career foundation for many of today’s leading researchers.
Conclusions
Congressionally Directed Medical Research Programs complement other federal and nonfederal sources of biomedical research funding and fill important research gaps through an evaluation of the funding landscape, identification of research gaps, and development of novel award mechanisms. The integration of survivors, patients, and their family members ensures that every aspect of the program management cycle balances scientific expertise with human perspective and has high impact on the patient community.
As new needs emerge, each research program designs an investment strategy to target areas most critically in need. The subsequent release of novel award mechanisms focuses research and enables an acceleration of science and/or leading researchers to the patient’s bedside. The CDMRP-funded discoveries have contributed to the development of new therapeutics, new diagnostics, and to changes in the standard of care exemplifying significant clinical impact and the innovative nature of these Congressional Special Interest Medical Research Programs.
To learn more about CDMRP or to receive funding notifications by e-mail, please visit http://cdmrp.army.mil.
Acknowledgments
The authors gratefully note the CDMRP Program Evaluation Steering Committee for critical review of the manuscript. Also acknowledged are CDMRP staff at large and Dr. Lisa Kinnard for support in this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Robinson DR, Kalyana-Sundaram S, Wu Y-I, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature Med. 2011;17(12):1646-1651.
1. Robinson DR, Kalyana-Sundaram S, Wu Y-I, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature Med. 2011;17(12):1646-1651.