User login
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis
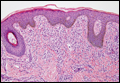
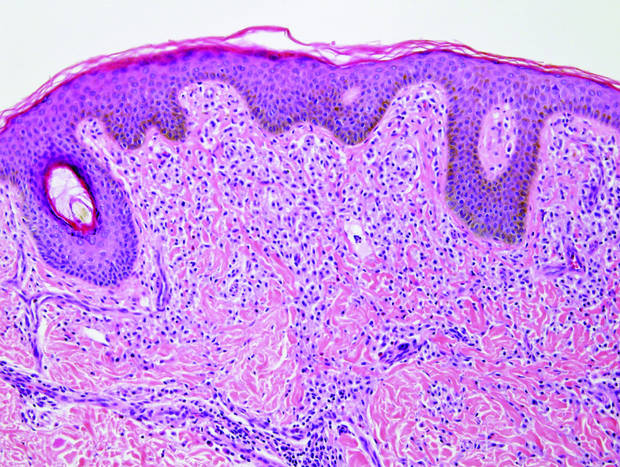 |
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |
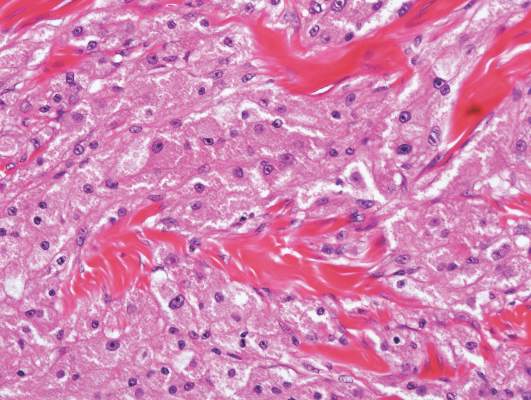
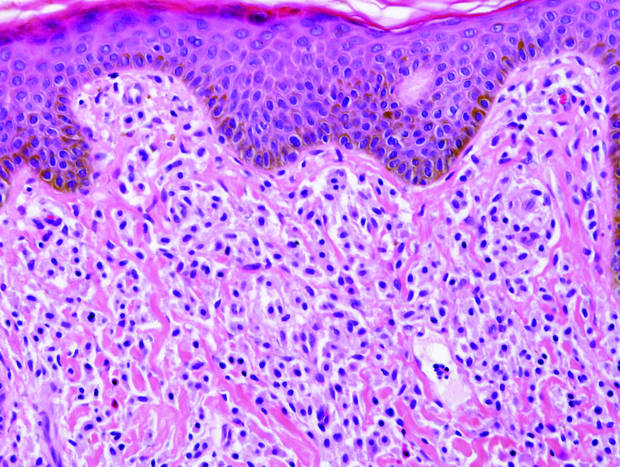 |
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.
 |
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |
 |
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21
 |
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |
 |
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis
 |
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |
 |
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.
 |
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |
 |
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21
 |
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |
 |
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis
 |
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |
 |
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.
 |
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |
 |
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21
 |
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |
 |
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.