User login
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
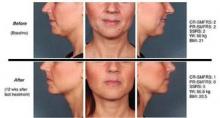
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE