User login
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
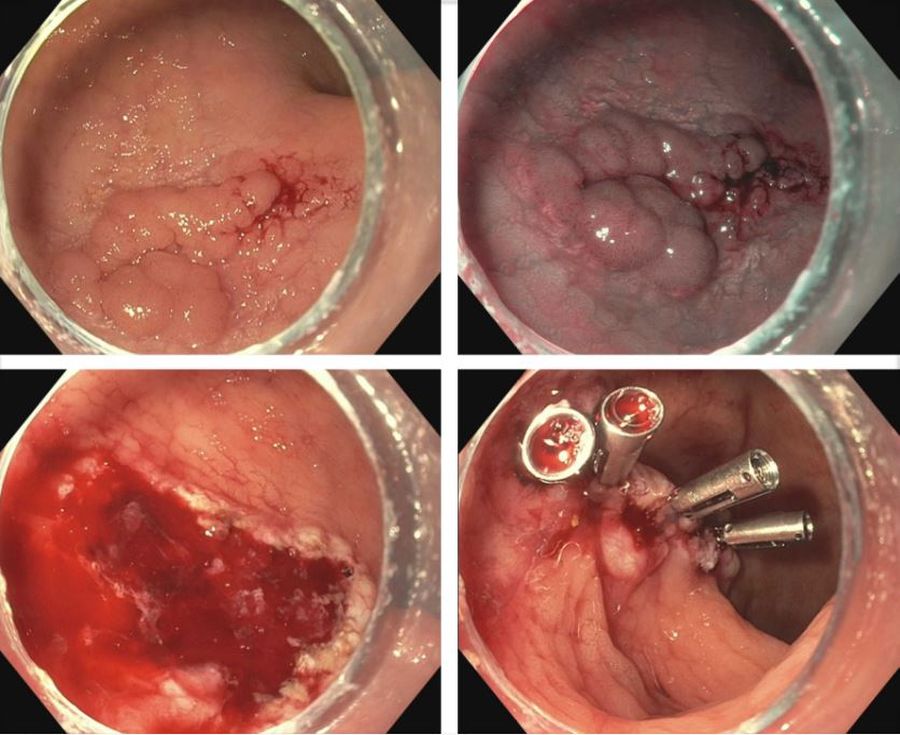
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16
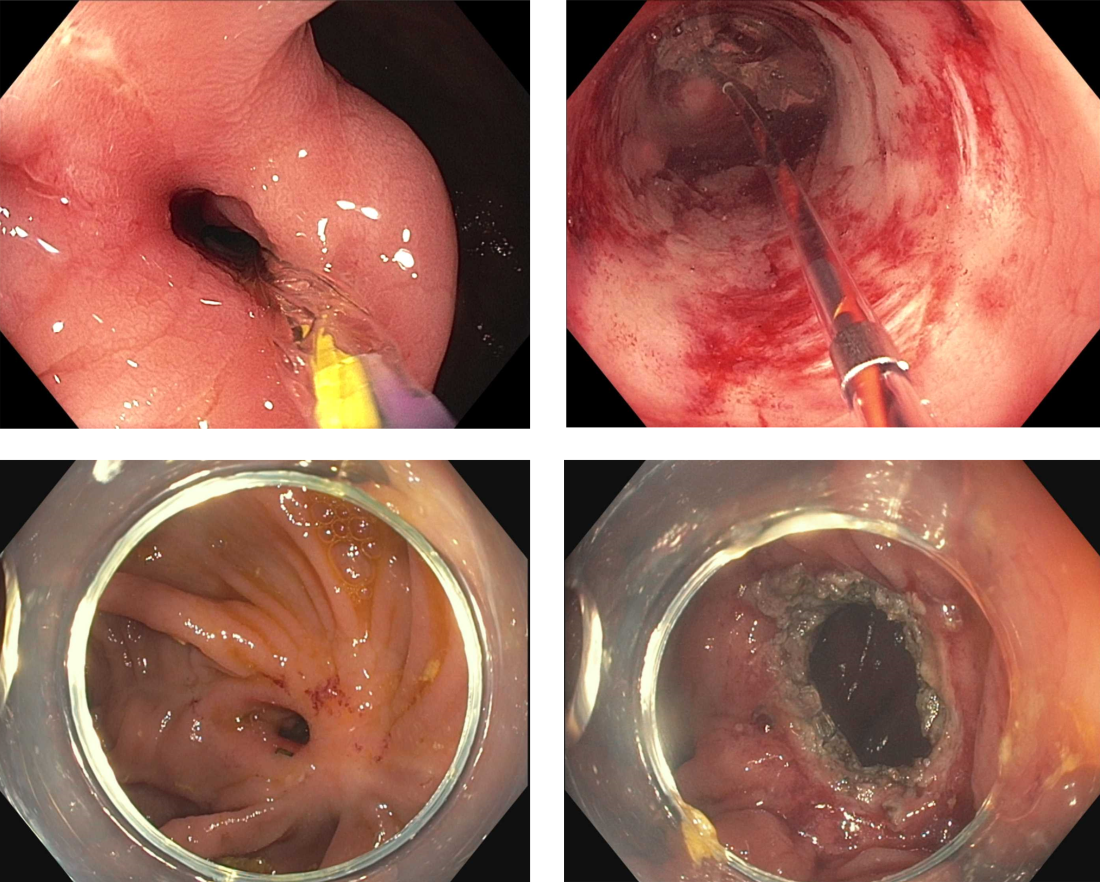
Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.