User login
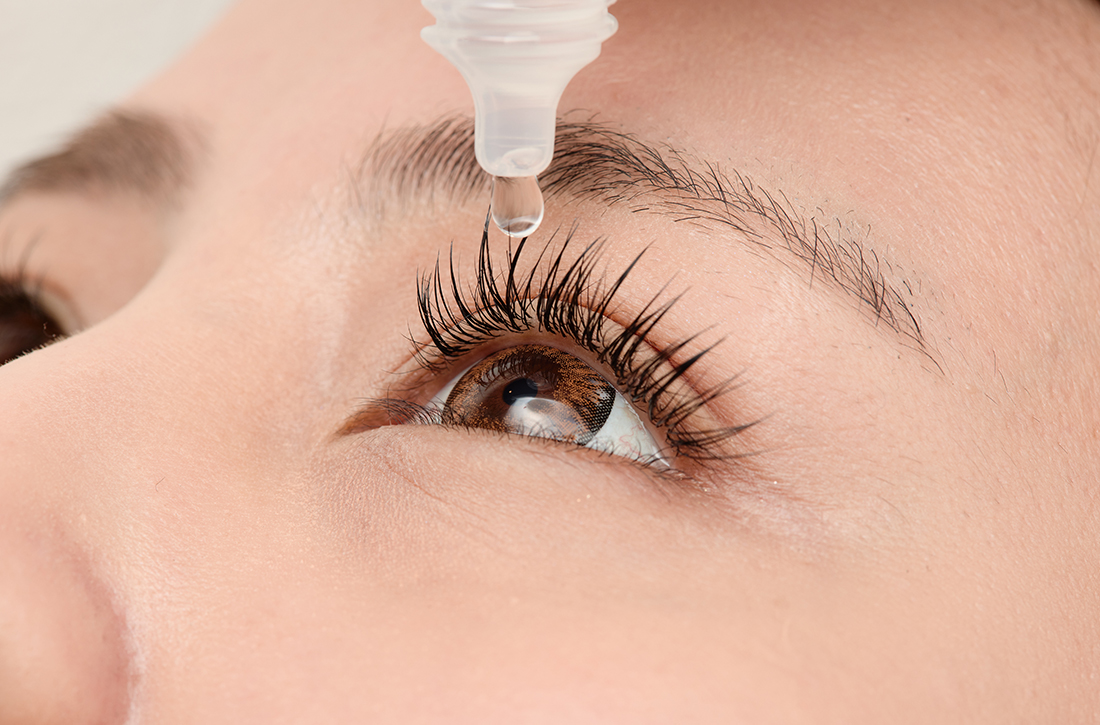
Migraine relief in 20 minutes using eyedrops?
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
PRACTICE CHANGER
Consider timolol maleate 0.5% eyedrops as a quick and effective abortive therapy for migraine.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.1
Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166.