User login
Are there perinatal benefits to pregnant patients after bariatric surgery?
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
The One Step test: The better diagnostic approach for gestational diabetes mellitus
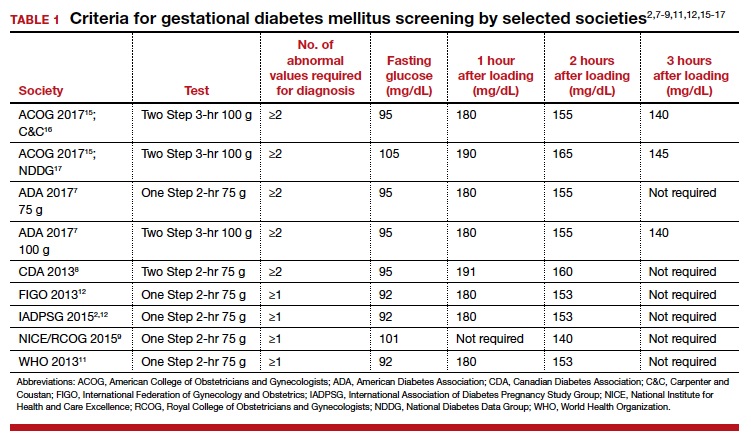
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19
Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
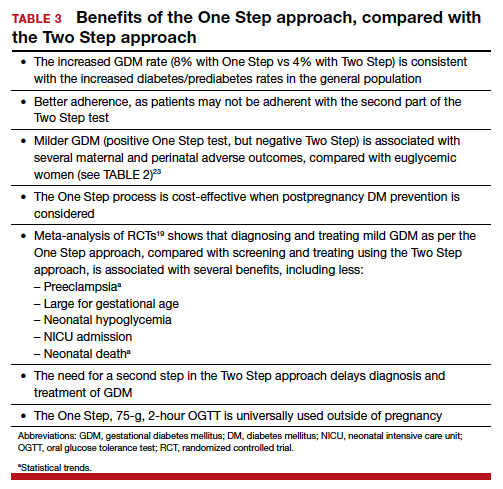
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19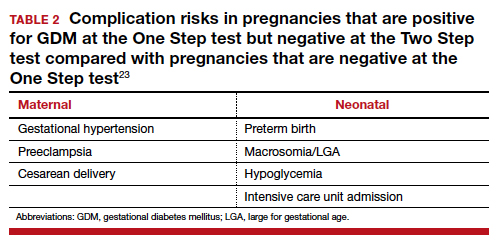
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Universal cervical length screening–saving babies lives
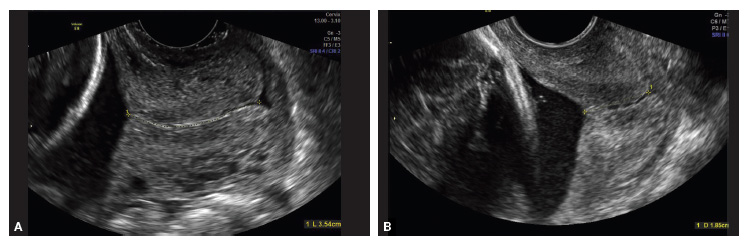
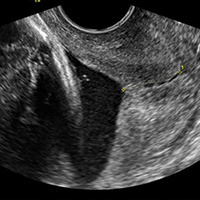
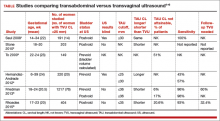
Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
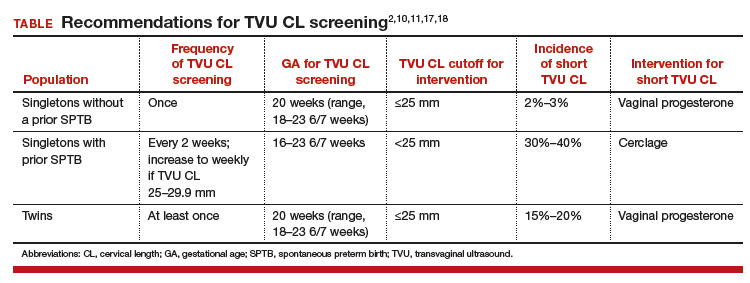
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).
Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).
Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).
Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
Can transabdominal ultrasound exclude short cervix?
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Infectious disease
Patrick Duff, MD (Update, June 2012)
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Does the rate of postcesarean maternal infection vary by uterine closure technique?
Vincenzo Berghella, MD (Examining the Evidence, February 2011)
The two most commonly utilized methods of skin closure after cesarean delivery are nonabsorbable metal staples and absorbable suture.1 A number of investigators have explored these methods of closure in regard to wound complications, pain perception, patient satisfaction, and physician assessment of cosmesis.2
A recent Cochrane meta-analysis of these studies revealed that there were no significant differences between these two methods with respect to wound infection, patient satisfaction, pain perception, or physician assessment of cosmesis.2 However, there was a significant difference between methods in terms of skin separation: Incisions closed with staples were almost four times as likely to be complicated by skin separation.2
Details of the trial
Participants had a viable pregnancy at 24 weeks’ gestation or beyond and were undergoing scheduled or unscheduled cesarean delivery. Of these, 198 women were randomly assigned to staples, and 200 were allocated to suture (Monocryl) for skin closure. Staples were removed 3 to 4 days after delivery for low transverse incisions and 7 to 10 days after delivery for vertical incisions.
Standardized physical examination of the wound was performed at hospital discharge (days 3–4) and 4 to 6 weeks postoperatively. The primary outcome was a composite of wound disruption or infection that occurred 4 to 6 weeks postoperatively; secondary outcomes included operative time, pain, cosmesis, and patient satisfaction with the scar.
Strengths of the trial include sample size
Of the studies that have been published to date, this trial by Figueroa and colleagues is the second largest to compare staples with suture for closure of cesarean skin incisions.
Another strength of this study is its intention-to-treat analysis and the low rate of patients who were lost to follow-up.
This study is similar to the largest study, by Basha and colleagues, that examined skin closure after cesarean, in that women undergoing cesarean delivery via vertical or low transverse incisions were allocated to closure of the skin with staples or absorbable (Monocryl) suture.3 In both studies, staples were removed 3 or 4 days after delivery, although Figueroa and colleagues specified that staples be removed on days 7 to 10 for women who had vertical incisions.3
A few weaknesses may limit generalizability of the findings
Figueroa and colleagues noted that women in their study received prophylactic antibiotics at the time of cord clamping, rather than preoperatively, although the latter approach now is considered more appropriate in terms of reducing wound morbidity.4
Another limitation: Enrollment was terminated early, after enrolling only approximately one-third of the intended sample size. Figueroa and colleagues explain that this decision was based on the findings of Basha and colleagues, which were published during active enrollment of the Figueroa study.3 Not only did Basha and colleagues report a higher incidence of wound complications than Figueroa and colleagues had used to calculate the required sample size, but the Basha study also concluded that sutures may be more optimal for skin closure with respect to skin separation.3
In the study by Figueroa and colleagues, the primary outcome was defined as a composite of wound disruption or infection. However, there was no specification as to length of skin dehiscence that would qualify as disruption—although the investigators did note that the difference in wound disruption remained statistically significant when analyses were limited to wounds involving disruption of more than 1 cm.
As have earlier studies, Figueroa and colleagues found that operative time was longer when sutures were used, compared with staples.
Most earlier studies that assessed cosmesis utilized the Physician Observer Scar Assessment Scale, but this study did not, so it is unclear whether the findings can be compared with prior investigations on this point.
For women undergoing cesarean delivery via low transverse incision, if staples are removed on day 3, the incidence of wound separation is higher—as both this study and earlier studies have demonstrated—so suture may be preferred. If, however, staples are removed later than day 3, data are insufficient to compare wound morbidity on the basis of skin closure techniques. (We recommend staple removal on day 5–10 for women of normal weight, and day 7–10 for women with a body mass index above 30 kg/m2). An additional randomized clinical trial is needed.
DHANYA MACKEEN, MD, MPH, AND
VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Mackeen AD, Devaraj T, Baxter JK. Cesarean skin closure p: a survey of obstetricians [published online ahead of print January 11 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2012.755509.
2. Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in cesarean section. Cochrane Database Syst Rev. 2012;11:CD003577.-doi:10.1002/14651858.CD003577.pub3.
3. Basha SL, Rochon ML, Quinones JN, Coassolo KM, Rust OA, Smulian JC. Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery. Am J Obstet Gynecol. 2010;203(3):285.e1-e8.doi:10.1016/j.ajog.2010.07.011.
4. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.doi:10.1016/j.ajog.2008.06.077.
Infectious disease
Patrick Duff, MD (Update, June 2012)
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Does the rate of postcesarean maternal infection vary by uterine closure technique?
Vincenzo Berghella, MD (Examining the Evidence, February 2011)
The two most commonly utilized methods of skin closure after cesarean delivery are nonabsorbable metal staples and absorbable suture.1 A number of investigators have explored these methods of closure in regard to wound complications, pain perception, patient satisfaction, and physician assessment of cosmesis.2
A recent Cochrane meta-analysis of these studies revealed that there were no significant differences between these two methods with respect to wound infection, patient satisfaction, pain perception, or physician assessment of cosmesis.2 However, there was a significant difference between methods in terms of skin separation: Incisions closed with staples were almost four times as likely to be complicated by skin separation.2
Details of the trial
Participants had a viable pregnancy at 24 weeks’ gestation or beyond and were undergoing scheduled or unscheduled cesarean delivery. Of these, 198 women were randomly assigned to staples, and 200 were allocated to suture (Monocryl) for skin closure. Staples were removed 3 to 4 days after delivery for low transverse incisions and 7 to 10 days after delivery for vertical incisions.
Standardized physical examination of the wound was performed at hospital discharge (days 3–4) and 4 to 6 weeks postoperatively. The primary outcome was a composite of wound disruption or infection that occurred 4 to 6 weeks postoperatively; secondary outcomes included operative time, pain, cosmesis, and patient satisfaction with the scar.
Strengths of the trial include sample size
Of the studies that have been published to date, this trial by Figueroa and colleagues is the second largest to compare staples with suture for closure of cesarean skin incisions.
Another strength of this study is its intention-to-treat analysis and the low rate of patients who were lost to follow-up.
This study is similar to the largest study, by Basha and colleagues, that examined skin closure after cesarean, in that women undergoing cesarean delivery via vertical or low transverse incisions were allocated to closure of the skin with staples or absorbable (Monocryl) suture.3 In both studies, staples were removed 3 or 4 days after delivery, although Figueroa and colleagues specified that staples be removed on days 7 to 10 for women who had vertical incisions.3
A few weaknesses may limit generalizability of the findings
Figueroa and colleagues noted that women in their study received prophylactic antibiotics at the time of cord clamping, rather than preoperatively, although the latter approach now is considered more appropriate in terms of reducing wound morbidity.4
Another limitation: Enrollment was terminated early, after enrolling only approximately one-third of the intended sample size. Figueroa and colleagues explain that this decision was based on the findings of Basha and colleagues, which were published during active enrollment of the Figueroa study.3 Not only did Basha and colleagues report a higher incidence of wound complications than Figueroa and colleagues had used to calculate the required sample size, but the Basha study also concluded that sutures may be more optimal for skin closure with respect to skin separation.3
In the study by Figueroa and colleagues, the primary outcome was defined as a composite of wound disruption or infection. However, there was no specification as to length of skin dehiscence that would qualify as disruption—although the investigators did note that the difference in wound disruption remained statistically significant when analyses were limited to wounds involving disruption of more than 1 cm.
As have earlier studies, Figueroa and colleagues found that operative time was longer when sutures were used, compared with staples.
Most earlier studies that assessed cosmesis utilized the Physician Observer Scar Assessment Scale, but this study did not, so it is unclear whether the findings can be compared with prior investigations on this point.
For women undergoing cesarean delivery via low transverse incision, if staples are removed on day 3, the incidence of wound separation is higher—as both this study and earlier studies have demonstrated—so suture may be preferred. If, however, staples are removed later than day 3, data are insufficient to compare wound morbidity on the basis of skin closure techniques. (We recommend staple removal on day 5–10 for women of normal weight, and day 7–10 for women with a body mass index above 30 kg/m2). An additional randomized clinical trial is needed.
DHANYA MACKEEN, MD, MPH, AND
VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
Infectious disease
Patrick Duff, MD (Update, June 2012)
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Does the rate of postcesarean maternal infection vary by uterine closure technique?
Vincenzo Berghella, MD (Examining the Evidence, February 2011)
The two most commonly utilized methods of skin closure after cesarean delivery are nonabsorbable metal staples and absorbable suture.1 A number of investigators have explored these methods of closure in regard to wound complications, pain perception, patient satisfaction, and physician assessment of cosmesis.2
A recent Cochrane meta-analysis of these studies revealed that there were no significant differences between these two methods with respect to wound infection, patient satisfaction, pain perception, or physician assessment of cosmesis.2 However, there was a significant difference between methods in terms of skin separation: Incisions closed with staples were almost four times as likely to be complicated by skin separation.2
Details of the trial
Participants had a viable pregnancy at 24 weeks’ gestation or beyond and were undergoing scheduled or unscheduled cesarean delivery. Of these, 198 women were randomly assigned to staples, and 200 were allocated to suture (Monocryl) for skin closure. Staples were removed 3 to 4 days after delivery for low transverse incisions and 7 to 10 days after delivery for vertical incisions.
Standardized physical examination of the wound was performed at hospital discharge (days 3–4) and 4 to 6 weeks postoperatively. The primary outcome was a composite of wound disruption or infection that occurred 4 to 6 weeks postoperatively; secondary outcomes included operative time, pain, cosmesis, and patient satisfaction with the scar.
Strengths of the trial include sample size
Of the studies that have been published to date, this trial by Figueroa and colleagues is the second largest to compare staples with suture for closure of cesarean skin incisions.
Another strength of this study is its intention-to-treat analysis and the low rate of patients who were lost to follow-up.
This study is similar to the largest study, by Basha and colleagues, that examined skin closure after cesarean, in that women undergoing cesarean delivery via vertical or low transverse incisions were allocated to closure of the skin with staples or absorbable (Monocryl) suture.3 In both studies, staples were removed 3 or 4 days after delivery, although Figueroa and colleagues specified that staples be removed on days 7 to 10 for women who had vertical incisions.3
A few weaknesses may limit generalizability of the findings
Figueroa and colleagues noted that women in their study received prophylactic antibiotics at the time of cord clamping, rather than preoperatively, although the latter approach now is considered more appropriate in terms of reducing wound morbidity.4
Another limitation: Enrollment was terminated early, after enrolling only approximately one-third of the intended sample size. Figueroa and colleagues explain that this decision was based on the findings of Basha and colleagues, which were published during active enrollment of the Figueroa study.3 Not only did Basha and colleagues report a higher incidence of wound complications than Figueroa and colleagues had used to calculate the required sample size, but the Basha study also concluded that sutures may be more optimal for skin closure with respect to skin separation.3
In the study by Figueroa and colleagues, the primary outcome was defined as a composite of wound disruption or infection. However, there was no specification as to length of skin dehiscence that would qualify as disruption—although the investigators did note that the difference in wound disruption remained statistically significant when analyses were limited to wounds involving disruption of more than 1 cm.
As have earlier studies, Figueroa and colleagues found that operative time was longer when sutures were used, compared with staples.
Most earlier studies that assessed cosmesis utilized the Physician Observer Scar Assessment Scale, but this study did not, so it is unclear whether the findings can be compared with prior investigations on this point.
For women undergoing cesarean delivery via low transverse incision, if staples are removed on day 3, the incidence of wound separation is higher—as both this study and earlier studies have demonstrated—so suture may be preferred. If, however, staples are removed later than day 3, data are insufficient to compare wound morbidity on the basis of skin closure techniques. (We recommend staple removal on day 5–10 for women of normal weight, and day 7–10 for women with a body mass index above 30 kg/m2). An additional randomized clinical trial is needed.
DHANYA MACKEEN, MD, MPH, AND
VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Mackeen AD, Devaraj T, Baxter JK. Cesarean skin closure p: a survey of obstetricians [published online ahead of print January 11 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2012.755509.
2. Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in cesarean section. Cochrane Database Syst Rev. 2012;11:CD003577.-doi:10.1002/14651858.CD003577.pub3.
3. Basha SL, Rochon ML, Quinones JN, Coassolo KM, Rust OA, Smulian JC. Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery. Am J Obstet Gynecol. 2010;203(3):285.e1-e8.doi:10.1016/j.ajog.2010.07.011.
4. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.doi:10.1016/j.ajog.2008.06.077.
1. Mackeen AD, Devaraj T, Baxter JK. Cesarean skin closure p: a survey of obstetricians [published online ahead of print January 11 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2012.755509.
2. Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in cesarean section. Cochrane Database Syst Rev. 2012;11:CD003577.-doi:10.1002/14651858.CD003577.pub3.
3. Basha SL, Rochon ML, Quinones JN, Coassolo KM, Rust OA, Smulian JC. Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery. Am J Obstet Gynecol. 2010;203(3):285.e1-e8.doi:10.1016/j.ajog.2010.07.011.
4. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301.e1-e6.doi:10.1016/j.ajog.2008.06.077.
Does the rate of postcesarean maternal infection vary by uterine closure technique?
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
More than one million cesarean deliveries are performed each year in the United States. That’s more than two cesarean deliveries every minute. Clearly, performing this most common of major surgeries safely and effectively is of the utmost importance.
The much-anticipated CAESAR study is a large randomized, controlled trial that assesses three technical aspects of cesarean delivery:
- single-layer versus double-layer uterine closure. The investigators define the former as approximation of both edges of the uterine incision using a single layer of sutures. Double-layer closure involves one set of sutures at the endometrial layer and an additional set of sutures at the serosal layer
- closure versus nonclosure of the pelvic peritoneum
- liberal versus restricted use of a subrectus sheath drain.
The primary outcome of this study is maternal infectious morbidity. Women who participated in the trial were all undergoing their first cesarean delivery, which was performed through the lower uterine segment. In addition, none of the women had a clear indication for any of the techniques explored in this study.
As in any trial, the findings of the CAESAR study should be interpreted in view of the totality of the literature. Other aspects of cesarean delivery have been summarized previously. 1
In the short term, the type of uterine closure doesn’t seem to matter
In the CAESAR trial, single-layer closure of the uterine incision was not associated with any effect on maternal infectious morbidity, compared with double-layer closure. In earlier randomized, controlled trials, single-layer closure was associated with shorter operative time, less blood loss, and less pain. 2
An important issue is the long-term effect of single-layer uterine closure, especially the incidence of uterine rupture in subsequent trials of labor, compared with double-layer closure. The CAESAR study did not report this outcome, but we hope that it will in the future, as it is one of the largest trials to explore uterine closure.
Closure of the peritoneum
In earlier randomized, controlled trials, non-closure of the pelvic peritoneum has been associated with shorter operative time and hospitalization, a lower rate of fever, and less need for analgesia. 3
In the CAESAR trial, nonclosure of the peritoneum was not associated with any effect on maternal infectious morbidity, compared with closure.
Use of a drain is best limited
In the CAESAR trial, restricted use of a subrectus sheath drain was not associated with any effect on maternal infectious morbidity, compared with liberal use. In earlier trials, drainage was not associated with any bene-fit. 4 Therefore, it seems preferable to limit use of these drains during cesarean delivery.
It is too soon for us to know the long-term effects of these cesarean delivery techniques, but neither single-layer nor double-layer uterine closure appears to affect the rate of maternal postoperative infection.
Nonclosure of the peritoneum is preferred to closure, based on Level I literature on this issue.
Liberal use of a subrectus sheath drain is of little benefit. Its use should be limited. —VINCENZO BERGHELLA, MD
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617.
2. Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;(3):CD004732.-
3. Bamigboye AA, Hofmeyr JG. Closure versus non-closure of the peritoneum at caesarean section. Cochrane Database Syst Rev. 1998;(1):CD000163.-doi: 10.1002/14651858. CD000163.
4. Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database Syst Rev. 2005;(1):CD004549.-doi: 10.1002/14651858.CD004549.pub2.