User login
Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis
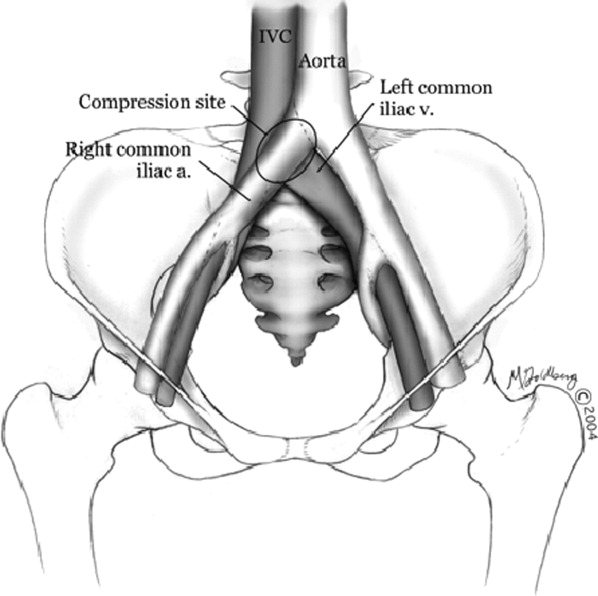
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.
11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
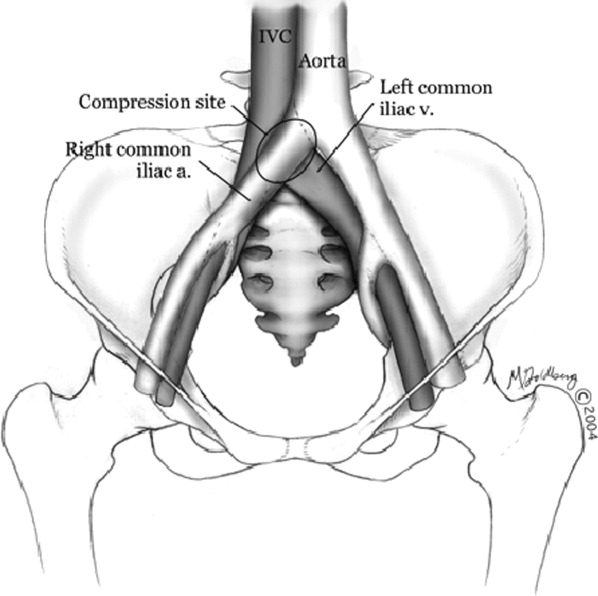
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.

11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.

11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
Cefepime: Underrecognized Cause of NCSE
Hospitalized patients with sepsis or severe nosocomial infections are frequently treated empirically with broad‐spectrum antibiotics. Cefepime hydrochloride, a fourth‐generation cephalosporin, is a common antibiotic of first choice. Its proconvulsant properties are well described in the literature,1, 2 but its importance as a potential cause of change in mental status is probably underestimated. We report a case of change in mental status related to nonconvulsive status epilepticus (NCSE) caused by the use of cefepime in an elderly, hospitalized patient. Our goal is to raise awareness about this uncommon and still underrecognized complication.
Case Report
A 72‐year‐old woman with stage III chronic kidney disease secondary to hypertension with a stable creatinine of 1.5 mg/dL (glomerular filtration rate (GFR) estimated by the modification of diet in renal disease (MDRD) at 36 mL/minute/1.73 m2) was admitted to the hospital for worsening of her chronic back pain. She had a past medical history significant for hyperlipidemia, asthma, and peripheral vascular disease, with breast cancer in remission since 1989. She had no history of seizures or cerebrovascular disease. Her medications were ibuprofen, oxycontin, cilastazol, acetaminophen/oxycodone, and an albuterol/empratropium inhaler. Her physical examination was remarkable only for decreased strength in the right lower extremity. Magnetic resonance imaging (MRI) of the lumbosacral spine showed signs consistent with an inflammatory process at the level of L4‐L5. A computed tomography (CT)‐guided biopsy was performed and confirmed a diagnosis of osteomyelitis on biopsy. Cultures from the biopsy grew Pseudomonas aeruginosa and treatment with intravenous cefepime at a dose of 1 g every 12 hours was initiated. Over the next 3 days, the patient had a gradual worsening of her mental status, leading to pronounced somnolence with occasional episodes of agitation during which she had no focal motor deficits. Her mental status declined to the point of unresponsiveness to simple verbal commands. She had not received any new medications other than cefepime. Her creatinine level was stable throughout this time period at 1.6 mg/dL. No other abnormalities were found on laboratory evaluation or on CT and MRI scans of the brain. An electroencephalogram (EEG) was markedly abnormal due to a generalized background slowing and disorganization with frequent bilateral paroxysmal epileptiform discharges, confirming the clinical diagnosis of subclinical generalized status epilepticus. Given that there were no other intrinsic neurological or metabolic reasons for this mental status change, and given that cefepime was the only new medication added before the patient started deteriorating, cefepime was discontinued and treatment for seizures was started with intravenous benzodiazepines. Over the next 2 days, her mental status returned to normal. She was soon discharged to a rehabilitation center.
Discussion
Beta‐lactam antibiotics have been described to induce seizures due to their direct and/or indirect inhibition of the gamma‐aminobutyric acid (GABA) system.1, 3 Previous experiments have shown a dose‐dependent effect on seizures, and suggest that the cephalosporin with the most pronounced proconvulsant effect is cefazolin.1, 3
Cefepime has been associated with neurological side effects such as headache, confusion, hallucinations, agitation, myoclonus, ataxia, seizures, and coma. Another underrecognized but critical side effect is NSCE. This is defined as seizure activity for more than 30 minutes, with cognitive and behavioral changes, but without convulsive clinical manifestations. This complication has been reported in the literature, but it is probably underrecognized.3‐7 The tendency for cefepime to produce more subclinical activity than the other cephalosporins is not well understood.
Cefepime is mainly eliminated though renal excretion (85%) and displays linear pharmacokinetic properties, thus its dose needs to be adjusted according to renal function. Consequently, in the case of renal dysfunction, accumulation of the drug is proportional to the degree of renal impairment. For NSCE, the most important risk factor is renal impairment, although cases in patients with normal kidney function have been described.36 Age, preexisting central nervous system (CNS) disease, sepsis, and cardiopulmonary bypass have also been reported as possible risk factors for NCSE.1
Cefepime can accumulate in the cerebrospinal fluid (CSF) in the setting of renal dysfunction, decreased protein‐binding capacity (as is sometimes seen in the elderly), and increased blood‐brain permeability in the setting of CNS infections. Accumulation of the drug in the CSF can lead to blockade of the GABA‐A receptor through a mechanism of competitive antagonism1, 8
The onset of NSCE varies between 1 and 16 days after initiation of cefepime therapy.3‐5, 7 It is frequently confused with delirium, since hospitalized patients treated with broad‐spectrum antibiotics such as cefepime frequently have other comorbidities and risk factors for delirium.
This can delay the diagnosis of NSCE due to a lack of awareness of this critical complication in the setting of renal dysfunction. In order to quantify the likelihood that the NSCE was related to cefepime and not to other causes, we calculated a Naranjo adverse drug events probability score, which consists of 9 questions on the relationship between the adverse event and the incriminated drug.9 Each answer is scored from 1 to +2 points. This score was designed to quantify the strength of the association between any adverse event and a pharmacological agent.
In our patient, the Naranjo score was 7 points, suggesting that the diagnosis of cefepime‐induced NCSE was probable.
The diagnosis of NCSE is made through a combination of a high index of clinical suspicion, specific findings on EEG, and improvement with withdrawal of the drug. Fatal outcomes have been reported.5, 6 Early and prompt recognition of the condition is crucial for the prevention of its morbidity and mortality.
The mainstay of treatment is prompt withdrawal of antibiotics and symptomatic treatment with benzodiazepines or barbiturates. Very severe cases with refractory seizures have been treated with hemodialysis. Phenytoin should be avoided as a treatment of this condition due to its lack of GABA‐agonist activity.
Conclusion
Cefepime can cause NCSE, predominantly in patients with renal dysfunction. Its frequency is probably underestimated in hospitalized patients with multiple comorbid conditions. Hospitalists should be aware of this unusual but critical relationship, especially in patients with renal failure. A high level of clinical suspicion and an emergency EEG are essential to obtain a prompt and accurate diagnosis.
- .Antibiotic‐induced convulsions.Crit Care Clin.1997;13:741‐762.
- ,.Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review.Pharmacotherapy.2006;26(8):1169‐1174.
- ,,,,.Relationship between structure and convulsant properties of some β‐lactams antibiotics following intracerebroventricular microinjections in rats.Antimicrob Agents Chemother.1995;39:232‐237.
- ,,,,,.Cefepime‐ and cefixime‐induced encephalopathy in a patient with normal renal function.Neurology.2005;65(11):1840.
- ,,, et al.Cefepime induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure.Intensive Care Med.2002;28:214‐217.
- ,,,.Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function.Epilepsy Behav.2006;8(1):312‐314.
- ,,, et al.Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure.Am J Med.2001;111(2):115‐119.
- ,,, et al.Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins.Neuropharmacology.2003;45(3):304‐314.
- ,,, et al.A method for estimating the probability of adverse drug reactions.Clin Pharmacol Ther.1981;30(2):239‐245.
Hospitalized patients with sepsis or severe nosocomial infections are frequently treated empirically with broad‐spectrum antibiotics. Cefepime hydrochloride, a fourth‐generation cephalosporin, is a common antibiotic of first choice. Its proconvulsant properties are well described in the literature,1, 2 but its importance as a potential cause of change in mental status is probably underestimated. We report a case of change in mental status related to nonconvulsive status epilepticus (NCSE) caused by the use of cefepime in an elderly, hospitalized patient. Our goal is to raise awareness about this uncommon and still underrecognized complication.
Case Report
A 72‐year‐old woman with stage III chronic kidney disease secondary to hypertension with a stable creatinine of 1.5 mg/dL (glomerular filtration rate (GFR) estimated by the modification of diet in renal disease (MDRD) at 36 mL/minute/1.73 m2) was admitted to the hospital for worsening of her chronic back pain. She had a past medical history significant for hyperlipidemia, asthma, and peripheral vascular disease, with breast cancer in remission since 1989. She had no history of seizures or cerebrovascular disease. Her medications were ibuprofen, oxycontin, cilastazol, acetaminophen/oxycodone, and an albuterol/empratropium inhaler. Her physical examination was remarkable only for decreased strength in the right lower extremity. Magnetic resonance imaging (MRI) of the lumbosacral spine showed signs consistent with an inflammatory process at the level of L4‐L5. A computed tomography (CT)‐guided biopsy was performed and confirmed a diagnosis of osteomyelitis on biopsy. Cultures from the biopsy grew Pseudomonas aeruginosa and treatment with intravenous cefepime at a dose of 1 g every 12 hours was initiated. Over the next 3 days, the patient had a gradual worsening of her mental status, leading to pronounced somnolence with occasional episodes of agitation during which she had no focal motor deficits. Her mental status declined to the point of unresponsiveness to simple verbal commands. She had not received any new medications other than cefepime. Her creatinine level was stable throughout this time period at 1.6 mg/dL. No other abnormalities were found on laboratory evaluation or on CT and MRI scans of the brain. An electroencephalogram (EEG) was markedly abnormal due to a generalized background slowing and disorganization with frequent bilateral paroxysmal epileptiform discharges, confirming the clinical diagnosis of subclinical generalized status epilepticus. Given that there were no other intrinsic neurological or metabolic reasons for this mental status change, and given that cefepime was the only new medication added before the patient started deteriorating, cefepime was discontinued and treatment for seizures was started with intravenous benzodiazepines. Over the next 2 days, her mental status returned to normal. She was soon discharged to a rehabilitation center.
Discussion
Beta‐lactam antibiotics have been described to induce seizures due to their direct and/or indirect inhibition of the gamma‐aminobutyric acid (GABA) system.1, 3 Previous experiments have shown a dose‐dependent effect on seizures, and suggest that the cephalosporin with the most pronounced proconvulsant effect is cefazolin.1, 3
Cefepime has been associated with neurological side effects such as headache, confusion, hallucinations, agitation, myoclonus, ataxia, seizures, and coma. Another underrecognized but critical side effect is NSCE. This is defined as seizure activity for more than 30 minutes, with cognitive and behavioral changes, but without convulsive clinical manifestations. This complication has been reported in the literature, but it is probably underrecognized.3‐7 The tendency for cefepime to produce more subclinical activity than the other cephalosporins is not well understood.
Cefepime is mainly eliminated though renal excretion (85%) and displays linear pharmacokinetic properties, thus its dose needs to be adjusted according to renal function. Consequently, in the case of renal dysfunction, accumulation of the drug is proportional to the degree of renal impairment. For NSCE, the most important risk factor is renal impairment, although cases in patients with normal kidney function have been described.36 Age, preexisting central nervous system (CNS) disease, sepsis, and cardiopulmonary bypass have also been reported as possible risk factors for NCSE.1
Cefepime can accumulate in the cerebrospinal fluid (CSF) in the setting of renal dysfunction, decreased protein‐binding capacity (as is sometimes seen in the elderly), and increased blood‐brain permeability in the setting of CNS infections. Accumulation of the drug in the CSF can lead to blockade of the GABA‐A receptor through a mechanism of competitive antagonism1, 8
The onset of NSCE varies between 1 and 16 days after initiation of cefepime therapy.3‐5, 7 It is frequently confused with delirium, since hospitalized patients treated with broad‐spectrum antibiotics such as cefepime frequently have other comorbidities and risk factors for delirium.
This can delay the diagnosis of NSCE due to a lack of awareness of this critical complication in the setting of renal dysfunction. In order to quantify the likelihood that the NSCE was related to cefepime and not to other causes, we calculated a Naranjo adverse drug events probability score, which consists of 9 questions on the relationship between the adverse event and the incriminated drug.9 Each answer is scored from 1 to +2 points. This score was designed to quantify the strength of the association between any adverse event and a pharmacological agent.
In our patient, the Naranjo score was 7 points, suggesting that the diagnosis of cefepime‐induced NCSE was probable.
The diagnosis of NCSE is made through a combination of a high index of clinical suspicion, specific findings on EEG, and improvement with withdrawal of the drug. Fatal outcomes have been reported.5, 6 Early and prompt recognition of the condition is crucial for the prevention of its morbidity and mortality.
The mainstay of treatment is prompt withdrawal of antibiotics and symptomatic treatment with benzodiazepines or barbiturates. Very severe cases with refractory seizures have been treated with hemodialysis. Phenytoin should be avoided as a treatment of this condition due to its lack of GABA‐agonist activity.
Conclusion
Cefepime can cause NCSE, predominantly in patients with renal dysfunction. Its frequency is probably underestimated in hospitalized patients with multiple comorbid conditions. Hospitalists should be aware of this unusual but critical relationship, especially in patients with renal failure. A high level of clinical suspicion and an emergency EEG are essential to obtain a prompt and accurate diagnosis.
Hospitalized patients with sepsis or severe nosocomial infections are frequently treated empirically with broad‐spectrum antibiotics. Cefepime hydrochloride, a fourth‐generation cephalosporin, is a common antibiotic of first choice. Its proconvulsant properties are well described in the literature,1, 2 but its importance as a potential cause of change in mental status is probably underestimated. We report a case of change in mental status related to nonconvulsive status epilepticus (NCSE) caused by the use of cefepime in an elderly, hospitalized patient. Our goal is to raise awareness about this uncommon and still underrecognized complication.
Case Report
A 72‐year‐old woman with stage III chronic kidney disease secondary to hypertension with a stable creatinine of 1.5 mg/dL (glomerular filtration rate (GFR) estimated by the modification of diet in renal disease (MDRD) at 36 mL/minute/1.73 m2) was admitted to the hospital for worsening of her chronic back pain. She had a past medical history significant for hyperlipidemia, asthma, and peripheral vascular disease, with breast cancer in remission since 1989. She had no history of seizures or cerebrovascular disease. Her medications were ibuprofen, oxycontin, cilastazol, acetaminophen/oxycodone, and an albuterol/empratropium inhaler. Her physical examination was remarkable only for decreased strength in the right lower extremity. Magnetic resonance imaging (MRI) of the lumbosacral spine showed signs consistent with an inflammatory process at the level of L4‐L5. A computed tomography (CT)‐guided biopsy was performed and confirmed a diagnosis of osteomyelitis on biopsy. Cultures from the biopsy grew Pseudomonas aeruginosa and treatment with intravenous cefepime at a dose of 1 g every 12 hours was initiated. Over the next 3 days, the patient had a gradual worsening of her mental status, leading to pronounced somnolence with occasional episodes of agitation during which she had no focal motor deficits. Her mental status declined to the point of unresponsiveness to simple verbal commands. She had not received any new medications other than cefepime. Her creatinine level was stable throughout this time period at 1.6 mg/dL. No other abnormalities were found on laboratory evaluation or on CT and MRI scans of the brain. An electroencephalogram (EEG) was markedly abnormal due to a generalized background slowing and disorganization with frequent bilateral paroxysmal epileptiform discharges, confirming the clinical diagnosis of subclinical generalized status epilepticus. Given that there were no other intrinsic neurological or metabolic reasons for this mental status change, and given that cefepime was the only new medication added before the patient started deteriorating, cefepime was discontinued and treatment for seizures was started with intravenous benzodiazepines. Over the next 2 days, her mental status returned to normal. She was soon discharged to a rehabilitation center.
Discussion
Beta‐lactam antibiotics have been described to induce seizures due to their direct and/or indirect inhibition of the gamma‐aminobutyric acid (GABA) system.1, 3 Previous experiments have shown a dose‐dependent effect on seizures, and suggest that the cephalosporin with the most pronounced proconvulsant effect is cefazolin.1, 3
Cefepime has been associated with neurological side effects such as headache, confusion, hallucinations, agitation, myoclonus, ataxia, seizures, and coma. Another underrecognized but critical side effect is NSCE. This is defined as seizure activity for more than 30 minutes, with cognitive and behavioral changes, but without convulsive clinical manifestations. This complication has been reported in the literature, but it is probably underrecognized.3‐7 The tendency for cefepime to produce more subclinical activity than the other cephalosporins is not well understood.
Cefepime is mainly eliminated though renal excretion (85%) and displays linear pharmacokinetic properties, thus its dose needs to be adjusted according to renal function. Consequently, in the case of renal dysfunction, accumulation of the drug is proportional to the degree of renal impairment. For NSCE, the most important risk factor is renal impairment, although cases in patients with normal kidney function have been described.36 Age, preexisting central nervous system (CNS) disease, sepsis, and cardiopulmonary bypass have also been reported as possible risk factors for NCSE.1
Cefepime can accumulate in the cerebrospinal fluid (CSF) in the setting of renal dysfunction, decreased protein‐binding capacity (as is sometimes seen in the elderly), and increased blood‐brain permeability in the setting of CNS infections. Accumulation of the drug in the CSF can lead to blockade of the GABA‐A receptor through a mechanism of competitive antagonism1, 8
The onset of NSCE varies between 1 and 16 days after initiation of cefepime therapy.3‐5, 7 It is frequently confused with delirium, since hospitalized patients treated with broad‐spectrum antibiotics such as cefepime frequently have other comorbidities and risk factors for delirium.
This can delay the diagnosis of NSCE due to a lack of awareness of this critical complication in the setting of renal dysfunction. In order to quantify the likelihood that the NSCE was related to cefepime and not to other causes, we calculated a Naranjo adverse drug events probability score, which consists of 9 questions on the relationship between the adverse event and the incriminated drug.9 Each answer is scored from 1 to +2 points. This score was designed to quantify the strength of the association between any adverse event and a pharmacological agent.
In our patient, the Naranjo score was 7 points, suggesting that the diagnosis of cefepime‐induced NCSE was probable.
The diagnosis of NCSE is made through a combination of a high index of clinical suspicion, specific findings on EEG, and improvement with withdrawal of the drug. Fatal outcomes have been reported.5, 6 Early and prompt recognition of the condition is crucial for the prevention of its morbidity and mortality.
The mainstay of treatment is prompt withdrawal of antibiotics and symptomatic treatment with benzodiazepines or barbiturates. Very severe cases with refractory seizures have been treated with hemodialysis. Phenytoin should be avoided as a treatment of this condition due to its lack of GABA‐agonist activity.
Conclusion
Cefepime can cause NCSE, predominantly in patients with renal dysfunction. Its frequency is probably underestimated in hospitalized patients with multiple comorbid conditions. Hospitalists should be aware of this unusual but critical relationship, especially in patients with renal failure. A high level of clinical suspicion and an emergency EEG are essential to obtain a prompt and accurate diagnosis.
- .Antibiotic‐induced convulsions.Crit Care Clin.1997;13:741‐762.
- ,.Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review.Pharmacotherapy.2006;26(8):1169‐1174.
- ,,,,.Relationship between structure and convulsant properties of some β‐lactams antibiotics following intracerebroventricular microinjections in rats.Antimicrob Agents Chemother.1995;39:232‐237.
- ,,,,,.Cefepime‐ and cefixime‐induced encephalopathy in a patient with normal renal function.Neurology.2005;65(11):1840.
- ,,, et al.Cefepime induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure.Intensive Care Med.2002;28:214‐217.
- ,,,.Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function.Epilepsy Behav.2006;8(1):312‐314.
- ,,, et al.Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure.Am J Med.2001;111(2):115‐119.
- ,,, et al.Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins.Neuropharmacology.2003;45(3):304‐314.
- ,,, et al.A method for estimating the probability of adverse drug reactions.Clin Pharmacol Ther.1981;30(2):239‐245.
- .Antibiotic‐induced convulsions.Crit Care Clin.1997;13:741‐762.
- ,.Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review.Pharmacotherapy.2006;26(8):1169‐1174.
- ,,,,.Relationship between structure and convulsant properties of some β‐lactams antibiotics following intracerebroventricular microinjections in rats.Antimicrob Agents Chemother.1995;39:232‐237.
- ,,,,,.Cefepime‐ and cefixime‐induced encephalopathy in a patient with normal renal function.Neurology.2005;65(11):1840.
- ,,, et al.Cefepime induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure.Intensive Care Med.2002;28:214‐217.
- ,,,.Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function.Epilepsy Behav.2006;8(1):312‐314.
- ,,, et al.Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure.Am J Med.2001;111(2):115‐119.
- ,,, et al.Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins.Neuropharmacology.2003;45(3):304‐314.
- ,,, et al.A method for estimating the probability of adverse drug reactions.Clin Pharmacol Ther.1981;30(2):239‐245.