User login
Pancreaticobiliary potpourri
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
Managing severe acute pancreatitis
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Routine early computed tomography to evaluate patients with severe acute pancreatitis wastes time and is necessary only if the diagnosis at presentation is not clearly consistent with acute pancreatitis.
- Optimum fluid resuscitation is now recommended, using lactated Ringer’s solution at a rate of 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
- Enteral feeding with either an elemental diet or a polymeric enteral formulation is first-line nutritional therapy.
- Antibiotics are no longer routinely used to prevent infection.
- Relief of abdominal compartment syndrome should be attempted by multiple means before resorting to open abdominal decompression.
Large gallstone ileus
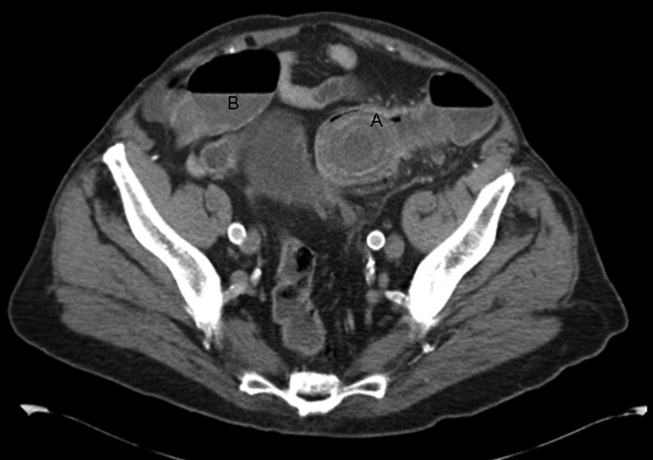
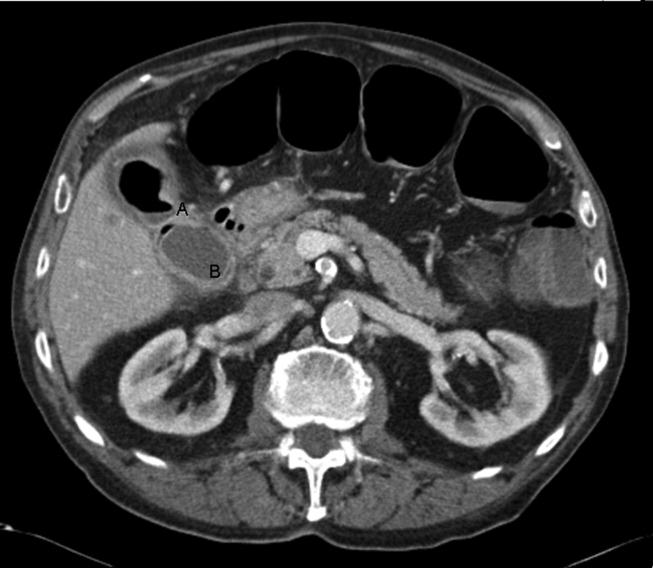
A 92‐year old man presented with a 5‐day history of obstipation, nausea, and vomiting. A computed tomography (CT) scan of the abdomen revealed a 4.1‐cm gallstone impacted in the sigmoid colon (Figure 1). The proximal colon was diffusely dilated in caliber consistent with obstruction (Figure 1B). The CT also showed a cholecystocolic fistula at the hepatic flexure of the colon (Figure 2) with an edematous gallbladder wall and a residual 3.8‐cm gallstone. Under colonoscopic guidance the stone was fragmented using intraluminal shock wave lithotripsy and other endoscopic techniques. The pieces were retrieved (Figure 3, shown reassembled). Cholecystectomy, common hepatic duct repair, and fistula takedown were electively performed to prevent recurrence.


Gallstone ileus is the mechanical impaction of gallstones within the gastrointestinal (GI) tract. It requires the formation of either a biliary‐enteric fistula or less often a choledocho‐enteric fistula. Usually the stone must be 2 cm or greater to cause obstruction.1 The site of obstruction is typically the terminal ileum or ileocecal valve because of the smaller diameter lumen and less active peristalsis. Although mortality rates approach 15%,2 this patient did remarkably well with early recognition, use of complex endoscopic removal, and avoidance of urgent laparotomy.
- ,.Gallstone ileus: a review of 1001 reported cases.Am Surg.1994;60:441–446.
- ,,,,,.[Gallstone Ileus: results of analysis of a series of 40 patients].Gastroenterol Hepatol.2001;24:489–494. In Spanish.
A 92‐year old man presented with a 5‐day history of obstipation, nausea, and vomiting. A computed tomography (CT) scan of the abdomen revealed a 4.1‐cm gallstone impacted in the sigmoid colon (Figure 1). The proximal colon was diffusely dilated in caliber consistent with obstruction (Figure 1B). The CT also showed a cholecystocolic fistula at the hepatic flexure of the colon (Figure 2) with an edematous gallbladder wall and a residual 3.8‐cm gallstone. Under colonoscopic guidance the stone was fragmented using intraluminal shock wave lithotripsy and other endoscopic techniques. The pieces were retrieved (Figure 3, shown reassembled). Cholecystectomy, common hepatic duct repair, and fistula takedown were electively performed to prevent recurrence.



Gallstone ileus is the mechanical impaction of gallstones within the gastrointestinal (GI) tract. It requires the formation of either a biliary‐enteric fistula or less often a choledocho‐enteric fistula. Usually the stone must be 2 cm or greater to cause obstruction.1 The site of obstruction is typically the terminal ileum or ileocecal valve because of the smaller diameter lumen and less active peristalsis. Although mortality rates approach 15%,2 this patient did remarkably well with early recognition, use of complex endoscopic removal, and avoidance of urgent laparotomy.
A 92‐year old man presented with a 5‐day history of obstipation, nausea, and vomiting. A computed tomography (CT) scan of the abdomen revealed a 4.1‐cm gallstone impacted in the sigmoid colon (Figure 1). The proximal colon was diffusely dilated in caliber consistent with obstruction (Figure 1B). The CT also showed a cholecystocolic fistula at the hepatic flexure of the colon (Figure 2) with an edematous gallbladder wall and a residual 3.8‐cm gallstone. Under colonoscopic guidance the stone was fragmented using intraluminal shock wave lithotripsy and other endoscopic techniques. The pieces were retrieved (Figure 3, shown reassembled). Cholecystectomy, common hepatic duct repair, and fistula takedown were electively performed to prevent recurrence.



Gallstone ileus is the mechanical impaction of gallstones within the gastrointestinal (GI) tract. It requires the formation of either a biliary‐enteric fistula or less often a choledocho‐enteric fistula. Usually the stone must be 2 cm or greater to cause obstruction.1 The site of obstruction is typically the terminal ileum or ileocecal valve because of the smaller diameter lumen and less active peristalsis. Although mortality rates approach 15%,2 this patient did remarkably well with early recognition, use of complex endoscopic removal, and avoidance of urgent laparotomy.
- ,.Gallstone ileus: a review of 1001 reported cases.Am Surg.1994;60:441–446.
- ,,,,,.[Gallstone Ileus: results of analysis of a series of 40 patients].Gastroenterol Hepatol.2001;24:489–494. In Spanish.
- ,.Gallstone ileus: a review of 1001 reported cases.Am Surg.1994;60:441–446.
- ,,,,,.[Gallstone Ileus: results of analysis of a series of 40 patients].Gastroenterol Hepatol.2001;24:489–494. In Spanish.