User login
How much does smoking cessation cut CHD risk?
Significantly. Patients with coronary heart disease (CHD) who refrain from smoking over a 2-year follow-up period decrease their relative risk (RR) for morbidity and mortality by about one third (strength of recommendation [SOR]: A, meta-analysis of 20 cohort studies). People who maintain abstinence after coronary artery bypass surgery are more likely to avoid angina, repeat revascularization, significant physical impairment, and CHD-related hospital admissions than patients who continue to smoke (SOR: A, 4 cohort studies with 1- to 20-year follow-up).
Evidence summary
The influence of cigarette smoking on the development of CHD has been well documented.1,2 RR ranges from 1.5 to 3, depending on variables such as age, sex, and quantity of tobacco used.3 Quitting smoking reduces overall mortality more than other forms of secondary prevention, including aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and cholesterol-lowering statins.3 In the wake of such evidence-based findings, the American Heart Association and American College of Cardiology Task Force developed clinical practice guidelines that recommend complete smoking cessation for secondary prevention of CHD in cardiac patients.4
0 cigarettes=lower mortality and morbidity
A Cochrane Heart Group meta-analysis examining all-cause CHD mortality in 20 cohort studies (n=12,603 patients), found a 36% reduction in mortality risk for CHD patients who quit smoking compared with those who didn’t (RR=0.64; 95% confidence interval [CI], 0.58-0.71).3 The review also noted a reduction in risk for nonfatal myocardial infarctions (RR=0.68; 95% CI, 0.57-0.82).3 The authors didn’t report how soon after smoking cessation mortality risk declined.
The authors acknowledge several limitations of the review, including the use of observational data and crude estimates, as well as potential publication bias and the misclassification of smoking status. Notably, however, their findings are consistent with the landmark prospective, community-based cohort Framingham Heart Study (N=1422), which indicates that smoking status predicts overall and morbidity-free survival at age 85.5
Smoking cessation has also been found to significantly affect morbidity among cardiac patients. Short-term benefits have been demonstrated in CHD patients after a myocardial infarction or coronary artery revascularization.6 Smoking status at 1-year follow-up was associated with a significant reduction in subsequent cardiac events (myocardial infarction, ischemic cerebrovascular event, revascularization, or death from CHD) when smokers who quit after an initial CHD event were compared with continuing smokers (odds ratio=0.71; 95% CI, 0.38-1.33).6
Going the distance is worth it
Regarding the role of extended abstinence on subsequent cardiac events, long-term quit status in post-coronary artery bypass graft (CABG) surgery patients has been found to predict decreased morbidity and lower rates of repeat revascularization surgery. Findings from the Coronary Artery Surgery Study show that, at 10-year follow-up, nonsmokers were more likely to be free of angina (54% of nonsmokers vs 42% of smokers; P=.02, NNT=8.3) and less likely to experience moderate to severe physical limitations (13% of non-smokers vs 24% of smokers; P=.0004; NNT=9.1). Nonsmokers also had fewer CHD-related admissions than smokers (2.6 vs 3.8; P<.0001).7
Another study found similar results at 20-year follow-up: Patients who had quit smoking underwent fewer repeat CABGs than smokers (RR=1.41; 95% CI, 1.02-1.94).8 The difference between post-CABG survival curves for quitters versus smokers increased from 3% at 5 years (98% vs 95%) to 15% at 15 years (70% vs 55%; P<.0001; NNT=6.7).8
Recommendations
The US Department of Health and Human Services recommends smoking cessation as an integral part of both primary and secondary prevention of CHD. Quitting reduces development of atherosclerosis and lowers the incidence of initial and recurrent myocardial infarction, thrombosis, cardiac arrhythmia, and death from cardiovascular causes.2
1. US Department of Health and Human Services. The Health Consequences of Smoking: What It Means to You. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. Available at: www.cdc.gov/tobacco/data_statistics/sgr/sgr_2004/00_pdfs/SGR2004_Whatitmeanstoyou.pdf. Accessed September 9, 2008.
2. US Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 90-8416;1990.
3. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;(1):CD003041.-
4. AHA ACC National Heart Lung and Blood Institute, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130-2139.
5. Terry DF, Pencina MJ, Vasan RS, et al. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;11:1944-1950.
6. Twardella D, Küpper-Nybelen J, Rothenbacher D, et al. Short-term benefit of smoking cessation in patients with coronary heart disease: estimates based on self-reported smoking data and serum cotinine measurements. Eur Heart J. 2004;25:2101-2108.
7. Cavender J, Rogers W, Fisher L, et al. for the CASS Investigators. Effects of smoking on survival and morbidity in patients randomized to medical or surgical therapy in the Coronary Artery Surgery Study (CASS): 10-year follow-up. J Am Coll Cardiol. 1992;20:287-294.
8. Van Domburg RT, Meeter K, van Berkel DF, et al. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. J Am Coll Cardiol. 2000;36:878-883.
Significantly. Patients with coronary heart disease (CHD) who refrain from smoking over a 2-year follow-up period decrease their relative risk (RR) for morbidity and mortality by about one third (strength of recommendation [SOR]: A, meta-analysis of 20 cohort studies). People who maintain abstinence after coronary artery bypass surgery are more likely to avoid angina, repeat revascularization, significant physical impairment, and CHD-related hospital admissions than patients who continue to smoke (SOR: A, 4 cohort studies with 1- to 20-year follow-up).
Evidence summary
The influence of cigarette smoking on the development of CHD has been well documented.1,2 RR ranges from 1.5 to 3, depending on variables such as age, sex, and quantity of tobacco used.3 Quitting smoking reduces overall mortality more than other forms of secondary prevention, including aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and cholesterol-lowering statins.3 In the wake of such evidence-based findings, the American Heart Association and American College of Cardiology Task Force developed clinical practice guidelines that recommend complete smoking cessation for secondary prevention of CHD in cardiac patients.4
0 cigarettes=lower mortality and morbidity
A Cochrane Heart Group meta-analysis examining all-cause CHD mortality in 20 cohort studies (n=12,603 patients), found a 36% reduction in mortality risk for CHD patients who quit smoking compared with those who didn’t (RR=0.64; 95% confidence interval [CI], 0.58-0.71).3 The review also noted a reduction in risk for nonfatal myocardial infarctions (RR=0.68; 95% CI, 0.57-0.82).3 The authors didn’t report how soon after smoking cessation mortality risk declined.
The authors acknowledge several limitations of the review, including the use of observational data and crude estimates, as well as potential publication bias and the misclassification of smoking status. Notably, however, their findings are consistent with the landmark prospective, community-based cohort Framingham Heart Study (N=1422), which indicates that smoking status predicts overall and morbidity-free survival at age 85.5
Smoking cessation has also been found to significantly affect morbidity among cardiac patients. Short-term benefits have been demonstrated in CHD patients after a myocardial infarction or coronary artery revascularization.6 Smoking status at 1-year follow-up was associated with a significant reduction in subsequent cardiac events (myocardial infarction, ischemic cerebrovascular event, revascularization, or death from CHD) when smokers who quit after an initial CHD event were compared with continuing smokers (odds ratio=0.71; 95% CI, 0.38-1.33).6
Going the distance is worth it
Regarding the role of extended abstinence on subsequent cardiac events, long-term quit status in post-coronary artery bypass graft (CABG) surgery patients has been found to predict decreased morbidity and lower rates of repeat revascularization surgery. Findings from the Coronary Artery Surgery Study show that, at 10-year follow-up, nonsmokers were more likely to be free of angina (54% of nonsmokers vs 42% of smokers; P=.02, NNT=8.3) and less likely to experience moderate to severe physical limitations (13% of non-smokers vs 24% of smokers; P=.0004; NNT=9.1). Nonsmokers also had fewer CHD-related admissions than smokers (2.6 vs 3.8; P<.0001).7
Another study found similar results at 20-year follow-up: Patients who had quit smoking underwent fewer repeat CABGs than smokers (RR=1.41; 95% CI, 1.02-1.94).8 The difference between post-CABG survival curves for quitters versus smokers increased from 3% at 5 years (98% vs 95%) to 15% at 15 years (70% vs 55%; P<.0001; NNT=6.7).8
Recommendations
The US Department of Health and Human Services recommends smoking cessation as an integral part of both primary and secondary prevention of CHD. Quitting reduces development of atherosclerosis and lowers the incidence of initial and recurrent myocardial infarction, thrombosis, cardiac arrhythmia, and death from cardiovascular causes.2
Significantly. Patients with coronary heart disease (CHD) who refrain from smoking over a 2-year follow-up period decrease their relative risk (RR) for morbidity and mortality by about one third (strength of recommendation [SOR]: A, meta-analysis of 20 cohort studies). People who maintain abstinence after coronary artery bypass surgery are more likely to avoid angina, repeat revascularization, significant physical impairment, and CHD-related hospital admissions than patients who continue to smoke (SOR: A, 4 cohort studies with 1- to 20-year follow-up).
Evidence summary
The influence of cigarette smoking on the development of CHD has been well documented.1,2 RR ranges from 1.5 to 3, depending on variables such as age, sex, and quantity of tobacco used.3 Quitting smoking reduces overall mortality more than other forms of secondary prevention, including aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and cholesterol-lowering statins.3 In the wake of such evidence-based findings, the American Heart Association and American College of Cardiology Task Force developed clinical practice guidelines that recommend complete smoking cessation for secondary prevention of CHD in cardiac patients.4
0 cigarettes=lower mortality and morbidity
A Cochrane Heart Group meta-analysis examining all-cause CHD mortality in 20 cohort studies (n=12,603 patients), found a 36% reduction in mortality risk for CHD patients who quit smoking compared with those who didn’t (RR=0.64; 95% confidence interval [CI], 0.58-0.71).3 The review also noted a reduction in risk for nonfatal myocardial infarctions (RR=0.68; 95% CI, 0.57-0.82).3 The authors didn’t report how soon after smoking cessation mortality risk declined.
The authors acknowledge several limitations of the review, including the use of observational data and crude estimates, as well as potential publication bias and the misclassification of smoking status. Notably, however, their findings are consistent with the landmark prospective, community-based cohort Framingham Heart Study (N=1422), which indicates that smoking status predicts overall and morbidity-free survival at age 85.5
Smoking cessation has also been found to significantly affect morbidity among cardiac patients. Short-term benefits have been demonstrated in CHD patients after a myocardial infarction or coronary artery revascularization.6 Smoking status at 1-year follow-up was associated with a significant reduction in subsequent cardiac events (myocardial infarction, ischemic cerebrovascular event, revascularization, or death from CHD) when smokers who quit after an initial CHD event were compared with continuing smokers (odds ratio=0.71; 95% CI, 0.38-1.33).6
Going the distance is worth it
Regarding the role of extended abstinence on subsequent cardiac events, long-term quit status in post-coronary artery bypass graft (CABG) surgery patients has been found to predict decreased morbidity and lower rates of repeat revascularization surgery. Findings from the Coronary Artery Surgery Study show that, at 10-year follow-up, nonsmokers were more likely to be free of angina (54% of nonsmokers vs 42% of smokers; P=.02, NNT=8.3) and less likely to experience moderate to severe physical limitations (13% of non-smokers vs 24% of smokers; P=.0004; NNT=9.1). Nonsmokers also had fewer CHD-related admissions than smokers (2.6 vs 3.8; P<.0001).7
Another study found similar results at 20-year follow-up: Patients who had quit smoking underwent fewer repeat CABGs than smokers (RR=1.41; 95% CI, 1.02-1.94).8 The difference between post-CABG survival curves for quitters versus smokers increased from 3% at 5 years (98% vs 95%) to 15% at 15 years (70% vs 55%; P<.0001; NNT=6.7).8
Recommendations
The US Department of Health and Human Services recommends smoking cessation as an integral part of both primary and secondary prevention of CHD. Quitting reduces development of atherosclerosis and lowers the incidence of initial and recurrent myocardial infarction, thrombosis, cardiac arrhythmia, and death from cardiovascular causes.2
1. US Department of Health and Human Services. The Health Consequences of Smoking: What It Means to You. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. Available at: www.cdc.gov/tobacco/data_statistics/sgr/sgr_2004/00_pdfs/SGR2004_Whatitmeanstoyou.pdf. Accessed September 9, 2008.
2. US Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 90-8416;1990.
3. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;(1):CD003041.-
4. AHA ACC National Heart Lung and Blood Institute, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130-2139.
5. Terry DF, Pencina MJ, Vasan RS, et al. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;11:1944-1950.
6. Twardella D, Küpper-Nybelen J, Rothenbacher D, et al. Short-term benefit of smoking cessation in patients with coronary heart disease: estimates based on self-reported smoking data and serum cotinine measurements. Eur Heart J. 2004;25:2101-2108.
7. Cavender J, Rogers W, Fisher L, et al. for the CASS Investigators. Effects of smoking on survival and morbidity in patients randomized to medical or surgical therapy in the Coronary Artery Surgery Study (CASS): 10-year follow-up. J Am Coll Cardiol. 1992;20:287-294.
8. Van Domburg RT, Meeter K, van Berkel DF, et al. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. J Am Coll Cardiol. 2000;36:878-883.
1. US Department of Health and Human Services. The Health Consequences of Smoking: What It Means to You. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. Available at: www.cdc.gov/tobacco/data_statistics/sgr/sgr_2004/00_pdfs/SGR2004_Whatitmeanstoyou.pdf. Accessed September 9, 2008.
2. US Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication No. (CDC) 90-8416;1990.
3. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;(1):CD003041.-
4. AHA ACC National Heart Lung and Blood Institute, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130-2139.
5. Terry DF, Pencina MJ, Vasan RS, et al. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;11:1944-1950.
6. Twardella D, Küpper-Nybelen J, Rothenbacher D, et al. Short-term benefit of smoking cessation in patients with coronary heart disease: estimates based on self-reported smoking data and serum cotinine measurements. Eur Heart J. 2004;25:2101-2108.
7. Cavender J, Rogers W, Fisher L, et al. for the CASS Investigators. Effects of smoking on survival and morbidity in patients randomized to medical or surgical therapy in the Coronary Artery Surgery Study (CASS): 10-year follow-up. J Am Coll Cardiol. 1992;20:287-294.
8. Van Domburg RT, Meeter K, van Berkel DF, et al. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. J Am Coll Cardiol. 2000;36:878-883.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best approach to goiter for euthyroid patients?
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
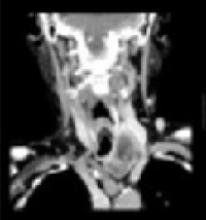
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
A detailed history and exam, confirmation of euthyroid status, and imaging when appropriate is the best approach to euthyroid patients with thyroid enlargement in regions where goiters are not endemic. Ultrasound imaging is recommended in any case of diagnostic uncertainty. Evaluate dominant or suspicious nodules further, while diffuse goiters without symptoms require no further evaluation and can be followed clinically (strength of recommendation [SOR]: C, expert opinion).
Suppressive therapy with levothyroxine can be used to decrease thyroid size for cosmetic reasons or in the case of mild local symptoms, although response is variable (SOR: B, based on small placebo-controlled trials). Patients with severe local symptoms should receive further evaluation and possible surgical management.
A patient with a euthyroid goiter; an opportunity to do no harm
John P. Langlois, MD
University of North Carolina School of Medicine, Chapel Hill
One of my patients is a 95-year-old woman who has spent most of the last 95 years taking very good care of herself. She has a small, slightly asymmetrical goiter that is probably only noticeable to her and to me (when she regularly calls my attention to it). One of the precepts of medicine is to “first do no harm,” and care of the patient with goiter is an excellent opportunity to practice this approach.
An initial ultrasound can give you good reassurance that the goiter is benign. Treatment of a goiter with thyroxine has questionable positive value and puts the patient at considerable risk for the problems of hyperthyroidism such as cardiac dysrhythmias and especially osteoporosis. So I keep tabs on her thyroid with a periodic thyroid-stimulating hormone (TSH) test and regular exams, and we spend a few moments at nearly every visit talking about how the best treatment can often be no treatment at all.
Evidence summary
Prevalence and types
In areas where iodine supplementation is routine, the prevalence of goiter is estimated to be 4% to 7%; however, autopsy studies show a 50% prevalence of nodules, most of which are multinodular goiter.1 Multinodular goiter is diagnosed in up to 5% of the general population and can be classified as euthyroid (“nontoxic”), hypothyroid, or hyperthyroid (“toxic”).1 Multinodular goiter is the most common diagnosis in cases of euthyroid goiter, but other conditions such as diffuse goiter (often idiopathic), thyroiditis, and neoplasms can also present in a euthyroid state.
Diagnosis: Exam and imaging
Studies show variable correlation between physical exam findings and findings on imaging studies. In a retrospective chart review, ultrasound findings differed from clinical exam findings in 63% of cases.2 Thyroid ultrasound is less expensive and less invasive than other imaging modalities, provides excellent visualization of thyroid structure and the nature of cysts and nodules, and allows for estimation of thyroid size.
Computed tomography and magnetic resonance imaging perform better for visualization of extension of thyroid tissue substernally and are also preferred for evaluation of the neck in cases of severe local complications of goiter, such as compressive symptoms.3
Abnormal left lobe of the thyroid gland
The malignant potential in multinodular goiter (2%–4%) is similar to that of solitary nodules (4%–6%).3 Therefore, any dominant nodule in a multinodular goiter should be evaluated the same way one would evaluate a solitary nodule.
Treatment and follow-up
Patients with a reassuring initial work-up can be followed clinically and should be assessed with serial clinical evaluations. No evidence could be found regarding optimal intervals for examination and testing; yearly exams and TSH testing are considered adequate by some experts.4
Because a few non-benign conditions such as thyroiditis and neoplasm can sometimes present in a euthyroid state, the clinician should be alert for any physical exam or laboratory changes. If any changes occur, then further workup is indicated.
Suppressive therapy with thyroxine is an option for decreasing thyroid size in euthyroid goiter, but this therapy remains controversial. One placebo-controlled trial of thyroid suppression in nontoxic multinodular goiter showed regression of thyroid size with suppressive therapy (58% reduction in size in treatment group vs 5% reduction in control group). However, not all goiters responded to this therapy, and the thyroid size returned to pretreatment size within 9 months of discontinuation of suppressive therapy.5
Many experts argue against the use of suppressive therapy in long-standing goiters, citing less response from these patients, along with concern about side effects and possible oversuppression, but the evidence in this area is limited. Patients who are treated with thyroxine should be followed for possible side effects of the medication, including arrhythmia and osteopenia, particularly in elderly patients and those who take the medication for long periods.
Recommendations of others
Guidelines from the American Association of Clinical Endocrinology’s Task Force on Thyroid Nodules, released in 2006, recommends ultrasound be used routinely in the case of multinodular goiter to assist with diagnosis, detect suspicious nodules that may require biopsy, and to serve as an objective baseline measure. This group recommends against use of suppression therapy in long-standing goiters.6
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
1. Day T, Chu A, Hoang K. Multinodular goiter. Otolaryngol Clin N Am 2003;36:35-54.
2. Marqusee E, Benson C, Frates M, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Int Med 2000;133:691-700.
3. Hurley D, Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin N Am 1996;29:527-540.
4. Supit E, Peiris A. Cost-effective management of thyroid nodules and nodular thyroid goiters. Southern Med J 2002;95:514-519.
5. Berghout A, et al. Comparison of placebo with L-thyroxine alone or carbimazole for treatment of sporadic non-toxic goiter. Lancet 1990;336:193-197.
6. AACE/AME Task Force on Thyroid Nodules. AACE/AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract 2006;12:53-102.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best duration of steroid therapy for contact dermatitis (rhus)?
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
Scant evidence exists for the best duration of steroid therapy for contact dermatitis due to plants (rhus). Review articles recommend 10 to 21 days of treatment with topical or oral corticosteroids for moderate to severe contact dermatitis due to plants (strength of recommendation [SOR]: C, based on review articles). The primary reason given for the duration of 2 to 3 weeks is to prevent rebound dermatitis.
Prescribe oral steroids for severe cases
Brian Crownover, MD, FAAFP, Lt Col, USAF, MC
US Air Force, Eglin Family Medicine Residency, Eglin Air Force Base, Eglin, Fla
Evidence for the best treatment of rhus dermatitis is negligible. Most recommendations stem from review articles and expert opinion. Rhus dermatitis is one example of a disorder for which we must fall back on our logic and personal experience. Since the painful itchy blisters and erythema from the oleoresin may take up to 1 week to appear, and because the rash may persist for more than 2 weeks, it makes sense to prescribe oral steroids in severe cases for longer than the usual 5- to 7-day burst. Habif, a popular dermatology text, suggests gradually tapering steroids from 60 to 10 mg over a 14-day course.1 In the absence of any randomized controlled trials (and remembering my patient who bounced back after I only gave 1 week of steroids), I will continue to prescribe 14 days of oral steroids.
Evidence summary
No published studies compare varying durations of treatment with steroids for contact dermatitis due to plants, including rhus. Many review articles refer to rebound dermatitis when using courses of oral steroids (such as Medrol dosepaks) for fewer than 14 days. One case report noted failure of a tapering dose over 5 days of oral methylprednisolone for treatment of poison ivy contact dermatitis.2
Most review articles recommend systemic steroids for severe poison ivy contact dermatitis, but these articles do not define “severe,” describe the taper, or give a definite length of treatment.3-5 One review recommends a tapering dose of oral prednisone to prevent rebound recurrence if the rash affects >25% of the body surface area, has severe blistering or itching, or significantly involves the face, hands, or genital area. That review suggests starting with oral prednisone 60 mg/d for 4 days, followed by a 10-day taper (50 mg/d for 2 days, 40 mg/d for 2 days, 30 mg/d for 2 days, 20 mg/d for 2 days, then 10 mg/d for 2 days).3
Another review recommends using systemic steroids for severe cases, defined as involvement of greater than 20% of total body surface area, bullae formation, or extensive facial involvement. That review recommends a starting dose of 1 mg/kg/d, or 40 to 60 mg/d in adults, followed by a 2- to 3-week taper of oral prednisone.4 A third review recommends using prednisone for children with allergic contact dermatitis involving more than 10% of the total body surface area.5
Recommendations from others
Guidelines for treatment of contact dermatitis published by the American Academy of Dermatology recommend topical treatment alone for mild cases of contact dermatitis, defined as “limited site of involvement, acute contact dermatitis when the offending agent has been removed, or chronic contact dermatitis with limited symptoms.” The guideline states that systemic treatment may be indicated to control itching or edema, or for moderate to severe cases. The systemic treatments listed include oral or intramuscular corticosteroids, but no discussion of duration is mentioned.6
UpToDate discusses avoidance of the offending substance for 2 to 4 weeks, use of topical corticosteroids of medium to strong potency for a limited time (without defining the duration), and use of systemic corticosteroids in severe cases, prescribing a course of prednisone at 40 mg daily for 4 to 6 days followed by 20 mg for 4 to 6 days.7
eMedicine states that although oral systemic steroids, with a taper of prednisone over 10 to 14 days, are the standard for severe toxicodendron dermatitis, some authors suggest high-potency steroid creams twice daily for a week, then daily for a week.8
ACP Medicine states that most cases of allergic contact dermatitis are “effectively managed without use of systemic corticosteroids,” but that “short courses of systemic corticosteroids are indicated for patients with severe vesiculobullous eruptions of the hands and feet or face,” without describing duration or dose.9
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
1. Habif TP. Clinical Dermatology. 4th ed. St. Louis, Mo: Mosby; 2004.
2. Ives TJ, Tepper RS. Failure of a tapering dose of oral methylprednisolone to treat reactions to poison ivy. JAMA 1991;266:1362.-
3. Brodell RT, Williams L. Taking the itch out of poison ivy: are you prescribing the right medication? Postgrad Med 1999;106:69-70.
4. Li LY, Cruz PD. Allergic contact dermatitis: Pathophysiology applied to future therapy. Dermatologic Therapy 2004;17:219-223.
5. Bruckner AL, Weston WL. Allergic contact dermatitis in children: A practical approach to management. Skin Therapy Letter 2002;7:3-5.
6. Rietschel RL, Adams RM, Daily AD. Guidelines of care for contact dermatitis. J Am Acad Dermatol 1995;32:109-113.
7. Overview of dermatitis. UpToDate [database]. Available at: www.uptodateonline.com/application/topic.asp?file=pri_derm/15769&type=A&selectedTitle=1~4. Accessed on January 10, 2006.
8. Stephanides SL. Plant poisoning, toxicodendron. E-Medicine [website]. Updated September 25, 2001. Available at www.emedicine.com/emerg/topic452.htm. Accessed on January 10, 2006.
9. Taylor JS. Contact dermatitis and related disorders. ACP Medicine. Updated September, 2005. Available at: www.acpmedicine.com/cgi-bin/publiccgi.pl?loginOP. Accessed on January 10, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
Who should have colposcopy?
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
Evidence-based answers from the Family Physicians Inquiries Network
In patients with a previous CVA, do antioxidants protect against subsequent stroke?
EVIDENCE-BASED ANSWER
Most recent randomized controlled clinical trials have not found a benefit in antioxidants (vitamin C, vitamin E, and/or beta-carotene) for preventing cardiovascular disease, including stroke. These recent clinical studies have not confirmed earlier observational studies that suggested a benefit. No studies have assessed only stroke patients and stroke outcomes. (Grade of recommendation: A, based on randomized controlled clinical trials and a systematic review of antioxidants and cardiovascular disease.)
Evidence summary
The Heart Outcomes Prevention Evaluation (HOPE) trial was a 4.5-year randomized controlled clinical trial of vitamin E or placebo in 9541 patients aged 55 years or older with a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes and other cardiovascular disease risk factors. No difference was noted between vitamin E and placebo for the outcomes of stroke, death, or other cardiac outcomes for these high-risk patients.1 In a randomized controlled clinical trial of 29,133 Finnish male smokers, the overall net stroke morbidity and mortality with antioxidants was not significantly different from placebo. However, a trend toward higher rates of subarachnoid hemorrhages was found (relative risk [RR] = 1.5; 95% confidence interval [CI], 0.97–2.32; numbers needed to harm [NNH] = 833), while the cerebral infarction rate was decreased (RR = 0.86; 95% CI, 0.75–0.99; numbers needed to treat = 239) by vitamin E. Beta-carotene increased intracerebral hemorrhage (RR = 1.61; 95% CI, 1.10–2.36; NNH = 546).2 Subsequent subgroup analysis showed a significant decrease in cerebral infarction (RR = 0.33; 95% CI, 0.14–0.78) without increasing subarachnoid hemorrhage in hypertensive, diabetic men taking vitamin E.3 Given the inherent methodological perils of subgroup analysis, this association requires further study before clinical implementation.
The Italian GISSI study of 11,324 patients with a recent myocardial infarction showed no effect of vitamin E on the combined outcomes of death, myocardial infarction, and stroke.4 In the Heart Protection Study, 20,536 adults between the ages of 40 and 80 years with cardiovascular disease, stroke, or diabetes were given vitamin E, vitamin C, beta-carotene, or placebo for 5 years. No significant differences were noted between vitamins and placebo in fatal or nonfatal stroke (RR = 0.99; 95% CI, 0.87–1.12).5
Although prior observational studies have hinted at a link between antioxidants and improved cardiovascular outcomes, the recently published Health Professionals Follow-up Study found no benefit to vitamin C or E in preventing strokes, based on the dietary assessment of 43,738 men, aged 40 to 75 years, who were not known to have cardiovascular disease or diabetes.6
Recommendations from others
The American Heart Association Science Advisory and Coordinating Committee commented on antioxidant use in 1999. While their emphasis was on coronary heart disease, they concluded that the general population should “consume a balanced diet with emphasis on antioxidant-rich fruits and vegetables and whole grains,” noting that “the absence of efficacy and safety data from randomized trials precludes the establishment of population-wide recommendations regarding vitamin E supplementation.”7 Some authors argue that the failure to demonstrate a benefit from antioxidants is due to inadequate antioxidant dosing, treatment length, or type of antioxidant.8
Read a Clinical Commentary by Dan Sontheimer, MD, MBA, online at http://www.FPIN.org.
1. Yusuf S, Dagenais G, Pogue J, et al. N Engl J Med 2000;342:154-60.
2. Leppala JM, Virtamo J, Fogelholm R, et al. Arterioscler Thromb Vasc Biol 2000;20:230-5.
3. Leppala JM, Virtamo J, Fogelholm R, et al. Arch Neurol 2000;57:1503-9.
4. GISSI Investigators. Lancet 1999;354:447-55.
5. MRC/BHF Heart Protection Study Group. Lancet 2002;360:23-33.
6. Ascherio A, Rimm EB, Hernan MA, et al. Ann Intern Med 1999;130:963-70.
7. Tribble DL. Circulation 1999;99:591-5.
8. Steinberg D, Witztum JL. Circulation 2002;105:2107-11.
EVIDENCE-BASED ANSWER
Most recent randomized controlled clinical trials have not found a benefit in antioxidants (vitamin C, vitamin E, and/or beta-carotene) for preventing cardiovascular disease, including stroke. These recent clinical studies have not confirmed earlier observational studies that suggested a benefit. No studies have assessed only stroke patients and stroke outcomes. (Grade of recommendation: A, based on randomized controlled clinical trials and a systematic review of antioxidants and cardiovascular disease.)
Evidence summary
The Heart Outcomes Prevention Evaluation (HOPE) trial was a 4.5-year randomized controlled clinical trial of vitamin E or placebo in 9541 patients aged 55 years or older with a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes and other cardiovascular disease risk factors. No difference was noted between vitamin E and placebo for the outcomes of stroke, death, or other cardiac outcomes for these high-risk patients.1 In a randomized controlled clinical trial of 29,133 Finnish male smokers, the overall net stroke morbidity and mortality with antioxidants was not significantly different from placebo. However, a trend toward higher rates of subarachnoid hemorrhages was found (relative risk [RR] = 1.5; 95% confidence interval [CI], 0.97–2.32; numbers needed to harm [NNH] = 833), while the cerebral infarction rate was decreased (RR = 0.86; 95% CI, 0.75–0.99; numbers needed to treat = 239) by vitamin E. Beta-carotene increased intracerebral hemorrhage (RR = 1.61; 95% CI, 1.10–2.36; NNH = 546).2 Subsequent subgroup analysis showed a significant decrease in cerebral infarction (RR = 0.33; 95% CI, 0.14–0.78) without increasing subarachnoid hemorrhage in hypertensive, diabetic men taking vitamin E.3 Given the inherent methodological perils of subgroup analysis, this association requires further study before clinical implementation.
The Italian GISSI study of 11,324 patients with a recent myocardial infarction showed no effect of vitamin E on the combined outcomes of death, myocardial infarction, and stroke.4 In the Heart Protection Study, 20,536 adults between the ages of 40 and 80 years with cardiovascular disease, stroke, or diabetes were given vitamin E, vitamin C, beta-carotene, or placebo for 5 years. No significant differences were noted between vitamins and placebo in fatal or nonfatal stroke (RR = 0.99; 95% CI, 0.87–1.12).5
Although prior observational studies have hinted at a link between antioxidants and improved cardiovascular outcomes, the recently published Health Professionals Follow-up Study found no benefit to vitamin C or E in preventing strokes, based on the dietary assessment of 43,738 men, aged 40 to 75 years, who were not known to have cardiovascular disease or diabetes.6
Recommendations from others
The American Heart Association Science Advisory and Coordinating Committee commented on antioxidant use in 1999. While their emphasis was on coronary heart disease, they concluded that the general population should “consume a balanced diet with emphasis on antioxidant-rich fruits and vegetables and whole grains,” noting that “the absence of efficacy and safety data from randomized trials precludes the establishment of population-wide recommendations regarding vitamin E supplementation.”7 Some authors argue that the failure to demonstrate a benefit from antioxidants is due to inadequate antioxidant dosing, treatment length, or type of antioxidant.8
Read a Clinical Commentary by Dan Sontheimer, MD, MBA, online at http://www.FPIN.org.
EVIDENCE-BASED ANSWER
Most recent randomized controlled clinical trials have not found a benefit in antioxidants (vitamin C, vitamin E, and/or beta-carotene) for preventing cardiovascular disease, including stroke. These recent clinical studies have not confirmed earlier observational studies that suggested a benefit. No studies have assessed only stroke patients and stroke outcomes. (Grade of recommendation: A, based on randomized controlled clinical trials and a systematic review of antioxidants and cardiovascular disease.)
Evidence summary
The Heart Outcomes Prevention Evaluation (HOPE) trial was a 4.5-year randomized controlled clinical trial of vitamin E or placebo in 9541 patients aged 55 years or older with a history of coronary artery disease, stroke, peripheral vascular disease, or diabetes and other cardiovascular disease risk factors. No difference was noted between vitamin E and placebo for the outcomes of stroke, death, or other cardiac outcomes for these high-risk patients.1 In a randomized controlled clinical trial of 29,133 Finnish male smokers, the overall net stroke morbidity and mortality with antioxidants was not significantly different from placebo. However, a trend toward higher rates of subarachnoid hemorrhages was found (relative risk [RR] = 1.5; 95% confidence interval [CI], 0.97–2.32; numbers needed to harm [NNH] = 833), while the cerebral infarction rate was decreased (RR = 0.86; 95% CI, 0.75–0.99; numbers needed to treat = 239) by vitamin E. Beta-carotene increased intracerebral hemorrhage (RR = 1.61; 95% CI, 1.10–2.36; NNH = 546).2 Subsequent subgroup analysis showed a significant decrease in cerebral infarction (RR = 0.33; 95% CI, 0.14–0.78) without increasing subarachnoid hemorrhage in hypertensive, diabetic men taking vitamin E.3 Given the inherent methodological perils of subgroup analysis, this association requires further study before clinical implementation.
The Italian GISSI study of 11,324 patients with a recent myocardial infarction showed no effect of vitamin E on the combined outcomes of death, myocardial infarction, and stroke.4 In the Heart Protection Study, 20,536 adults between the ages of 40 and 80 years with cardiovascular disease, stroke, or diabetes were given vitamin E, vitamin C, beta-carotene, or placebo for 5 years. No significant differences were noted between vitamins and placebo in fatal or nonfatal stroke (RR = 0.99; 95% CI, 0.87–1.12).5
Although prior observational studies have hinted at a link between antioxidants and improved cardiovascular outcomes, the recently published Health Professionals Follow-up Study found no benefit to vitamin C or E in preventing strokes, based on the dietary assessment of 43,738 men, aged 40 to 75 years, who were not known to have cardiovascular disease or diabetes.6
Recommendations from others
The American Heart Association Science Advisory and Coordinating Committee commented on antioxidant use in 1999. While their emphasis was on coronary heart disease, they concluded that the general population should “consume a balanced diet with emphasis on antioxidant-rich fruits and vegetables and whole grains,” noting that “the absence of efficacy and safety data from randomized trials precludes the establishment of population-wide recommendations regarding vitamin E supplementation.”7 Some authors argue that the failure to demonstrate a benefit from antioxidants is due to inadequate antioxidant dosing, treatment length, or type of antioxidant.8
Read a Clinical Commentary by Dan Sontheimer, MD, MBA, online at http://www.FPIN.org.
1. Yusuf S, Dagenais G, Pogue J, et al. N Engl J Med 2000;342:154-60.
2. Leppala JM, Virtamo J, Fogelholm R, et al. Arterioscler Thromb Vasc Biol 2000;20:230-5.
3. Leppala JM, Virtamo J, Fogelholm R, et al. Arch Neurol 2000;57:1503-9.
4. GISSI Investigators. Lancet 1999;354:447-55.
5. MRC/BHF Heart Protection Study Group. Lancet 2002;360:23-33.
6. Ascherio A, Rimm EB, Hernan MA, et al. Ann Intern Med 1999;130:963-70.
7. Tribble DL. Circulation 1999;99:591-5.
8. Steinberg D, Witztum JL. Circulation 2002;105:2107-11.
1. Yusuf S, Dagenais G, Pogue J, et al. N Engl J Med 2000;342:154-60.
2. Leppala JM, Virtamo J, Fogelholm R, et al. Arterioscler Thromb Vasc Biol 2000;20:230-5.
3. Leppala JM, Virtamo J, Fogelholm R, et al. Arch Neurol 2000;57:1503-9.
4. GISSI Investigators. Lancet 1999;354:447-55.
5. MRC/BHF Heart Protection Study Group. Lancet 2002;360:23-33.
6. Ascherio A, Rimm EB, Hernan MA, et al. Ann Intern Med 1999;130:963-70.
7. Tribble DL. Circulation 1999;99:591-5.
8. Steinberg D, Witztum JL. Circulation 2002;105:2107-11.
Evidence-based answers from the Family Physicians Inquiries Network
What are the most effective interventions to reduce childhood obesity?
EVIDENCE-BASED ANSWER
Efforts to increase physical activity or decrease sedentary activities have shown some short-term benefit, and adding dietary changes may be more effective. Aiming interventions at parents, intensive family therapy, comprehensive school-based programs, and selecting motivated children for subspecialty care may improve success. (Grade of recommendation: B, based on poor-quality randomized controlled trials [RCTs] and heterogeneous systematic reviews.) Other potentially effective short-term strategies include screening with body mass index (BMI) for age (grade of recommendation: C, extrapolation from cohort studies and ecological research) or dietary counseling (grade of recommendation: D, conflicting poor-quality RCTs). No drugs are currently approved for pediatric obesity therapy in the United States.
Evidence summary
Pediatric obesity increases the risk of adverse outcomes in adulthood, independent of adult BMI.1 Trials aiming to reduce childhood obesity suffer from serious methodological constraints. No long-term (> 2 years) evidence is available. Many apparently efficacious interventions are beyond the scope of primary care physicians. A detailed summary is available online at http://www.FPIN.org.
Several studies have examined the value of isolated changes in either diet or activity level. Randomized controlled trials and retrospective cohort studies of dietary advice alone show short-term efficacy (weeks to months).1 Most involve intensive subspecialty care for extremely obese children who are 120% to 140% over their ideal body weight. Trials without careful selection of motivated children had dropout rates up to 87%. One Italian RCT showed a 12% reduction in the number of obese children in schools receiving multimedia dietary advice compared with a 5% to 6% increase in those schools receiving only written or no advice.2 Several RCTs reduced obesity by introducing or improving school-based physical activity.1 Two RCTs that discouraged sedentary activity through counseling or school-based programs also reduced obesity.3,4
Two larger trials integrated diet and exercise advice into school curricula. One study emphasized improving school menus, but no difference was observed because children compensated by overeating at home.5 The other emphasized reducing sedentary activities and found lower obesity rates in the intervention schools, but only for girls.6 A third trial provided family-based dietary and behavior counseling, but emphasized either increasing physical activity or decreasing sedentary activity.7 Both strategies resulted in reduced obesity compared with controls. A systematic review supported the combined approach of these trials, finding diet and exercise interventions superior to diet interventions alone.8
Some evidence supports focusing on the family rather than just the child. A systematic review found that family therapy prevented pediatric obesity.9 An RCT found that focusing on parents as the sole change agent was superior to targeting the child.10
Recommendations from others
Expert consensus promotes screening because of obesity’s increasing incidence and associated morbidity and mortality.11 The Maternal and Child Health Bureau recommends a primary goal of healthy eating and activity. They recommend treating when the body mass index is >95th percentile, and assessing the child and family’s willingness to change. Primary strategies are to begin early, involve the family, promote parenting skills, and increase activity and reduce high-calorie food intake. They also recommend ongoing support to maintain weight loss.9
Clinical Commentary by Tess Bobo, MD, at http://www.fpin.org.
1. Campbell K, Waters E, O’Meara S, et al. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
2. Simonetti D’Arca A, Tarsitani G, Cairella M, et al. Public Health 1986;100:166-73.
3. Robinson TN. JAMA 1999;282:1561-7.
4. Faith MS, Berman N, Heo M, et al. Pediatrics 2001;107:1043-8.
5. Donnelly JE, Jacobsen DJ, Whatley JE, et al. Obes Res 1996;4:229-43.
6. Gortmaker SL, Peterson K, Wiecha J, et al. Arch Pediatr Adolesc Med 1999;153:409-18.
7. Epstein LH, Paluch RA, Gordy CC, Dorn J. Arch Pediatr Adolesc Med 2000;154:220-6.
8. Epstein LH, Goldfield GS. Med Sci Sports Exerc 1999;31(suppl):S553-9.
9. Glenny AM, O’Meara S. CRD Report 1997;10:1-149.
10. Golan M, Weizman A, Apter A, Fainaru M. Am J Clin Nutr 1998;67:1130-5.
11. Barlow SE, Dietz WH. Pediatrics 1998;102:E29.-
EVIDENCE-BASED ANSWER
Efforts to increase physical activity or decrease sedentary activities have shown some short-term benefit, and adding dietary changes may be more effective. Aiming interventions at parents, intensive family therapy, comprehensive school-based programs, and selecting motivated children for subspecialty care may improve success. (Grade of recommendation: B, based on poor-quality randomized controlled trials [RCTs] and heterogeneous systematic reviews.) Other potentially effective short-term strategies include screening with body mass index (BMI) for age (grade of recommendation: C, extrapolation from cohort studies and ecological research) or dietary counseling (grade of recommendation: D, conflicting poor-quality RCTs). No drugs are currently approved for pediatric obesity therapy in the United States.
Evidence summary
Pediatric obesity increases the risk of adverse outcomes in adulthood, independent of adult BMI.1 Trials aiming to reduce childhood obesity suffer from serious methodological constraints. No long-term (> 2 years) evidence is available. Many apparently efficacious interventions are beyond the scope of primary care physicians. A detailed summary is available online at http://www.FPIN.org.
Several studies have examined the value of isolated changes in either diet or activity level. Randomized controlled trials and retrospective cohort studies of dietary advice alone show short-term efficacy (weeks to months).1 Most involve intensive subspecialty care for extremely obese children who are 120% to 140% over their ideal body weight. Trials without careful selection of motivated children had dropout rates up to 87%. One Italian RCT showed a 12% reduction in the number of obese children in schools receiving multimedia dietary advice compared with a 5% to 6% increase in those schools receiving only written or no advice.2 Several RCTs reduced obesity by introducing or improving school-based physical activity.1 Two RCTs that discouraged sedentary activity through counseling or school-based programs also reduced obesity.3,4
Two larger trials integrated diet and exercise advice into school curricula. One study emphasized improving school menus, but no difference was observed because children compensated by overeating at home.5 The other emphasized reducing sedentary activities and found lower obesity rates in the intervention schools, but only for girls.6 A third trial provided family-based dietary and behavior counseling, but emphasized either increasing physical activity or decreasing sedentary activity.7 Both strategies resulted in reduced obesity compared with controls. A systematic review supported the combined approach of these trials, finding diet and exercise interventions superior to diet interventions alone.8
Some evidence supports focusing on the family rather than just the child. A systematic review found that family therapy prevented pediatric obesity.9 An RCT found that focusing on parents as the sole change agent was superior to targeting the child.10
Recommendations from others
Expert consensus promotes screening because of obesity’s increasing incidence and associated morbidity and mortality.11 The Maternal and Child Health Bureau recommends a primary goal of healthy eating and activity. They recommend treating when the body mass index is >95th percentile, and assessing the child and family’s willingness to change. Primary strategies are to begin early, involve the family, promote parenting skills, and increase activity and reduce high-calorie food intake. They also recommend ongoing support to maintain weight loss.9
Clinical Commentary by Tess Bobo, MD, at http://www.fpin.org.
EVIDENCE-BASED ANSWER
Efforts to increase physical activity or decrease sedentary activities have shown some short-term benefit, and adding dietary changes may be more effective. Aiming interventions at parents, intensive family therapy, comprehensive school-based programs, and selecting motivated children for subspecialty care may improve success. (Grade of recommendation: B, based on poor-quality randomized controlled trials [RCTs] and heterogeneous systematic reviews.) Other potentially effective short-term strategies include screening with body mass index (BMI) for age (grade of recommendation: C, extrapolation from cohort studies and ecological research) or dietary counseling (grade of recommendation: D, conflicting poor-quality RCTs). No drugs are currently approved for pediatric obesity therapy in the United States.
Evidence summary
Pediatric obesity increases the risk of adverse outcomes in adulthood, independent of adult BMI.1 Trials aiming to reduce childhood obesity suffer from serious methodological constraints. No long-term (> 2 years) evidence is available. Many apparently efficacious interventions are beyond the scope of primary care physicians. A detailed summary is available online at http://www.FPIN.org.
Several studies have examined the value of isolated changes in either diet or activity level. Randomized controlled trials and retrospective cohort studies of dietary advice alone show short-term efficacy (weeks to months).1 Most involve intensive subspecialty care for extremely obese children who are 120% to 140% over their ideal body weight. Trials without careful selection of motivated children had dropout rates up to 87%. One Italian RCT showed a 12% reduction in the number of obese children in schools receiving multimedia dietary advice compared with a 5% to 6% increase in those schools receiving only written or no advice.2 Several RCTs reduced obesity by introducing or improving school-based physical activity.1 Two RCTs that discouraged sedentary activity through counseling or school-based programs also reduced obesity.3,4
Two larger trials integrated diet and exercise advice into school curricula. One study emphasized improving school menus, but no difference was observed because children compensated by overeating at home.5 The other emphasized reducing sedentary activities and found lower obesity rates in the intervention schools, but only for girls.6 A third trial provided family-based dietary and behavior counseling, but emphasized either increasing physical activity or decreasing sedentary activity.7 Both strategies resulted in reduced obesity compared with controls. A systematic review supported the combined approach of these trials, finding diet and exercise interventions superior to diet interventions alone.8
Some evidence supports focusing on the family rather than just the child. A systematic review found that family therapy prevented pediatric obesity.9 An RCT found that focusing on parents as the sole change agent was superior to targeting the child.10
Recommendations from others
Expert consensus promotes screening because of obesity’s increasing incidence and associated morbidity and mortality.11 The Maternal and Child Health Bureau recommends a primary goal of healthy eating and activity. They recommend treating when the body mass index is >95th percentile, and assessing the child and family’s willingness to change. Primary strategies are to begin early, involve the family, promote parenting skills, and increase activity and reduce high-calorie food intake. They also recommend ongoing support to maintain weight loss.9
Clinical Commentary by Tess Bobo, MD, at http://www.fpin.org.
1. Campbell K, Waters E, O’Meara S, et al. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
2. Simonetti D’Arca A, Tarsitani G, Cairella M, et al. Public Health 1986;100:166-73.
3. Robinson TN. JAMA 1999;282:1561-7.
4. Faith MS, Berman N, Heo M, et al. Pediatrics 2001;107:1043-8.
5. Donnelly JE, Jacobsen DJ, Whatley JE, et al. Obes Res 1996;4:229-43.
6. Gortmaker SL, Peterson K, Wiecha J, et al. Arch Pediatr Adolesc Med 1999;153:409-18.
7. Epstein LH, Paluch RA, Gordy CC, Dorn J. Arch Pediatr Adolesc Med 2000;154:220-6.
8. Epstein LH, Goldfield GS. Med Sci Sports Exerc 1999;31(suppl):S553-9.
9. Glenny AM, O’Meara S. CRD Report 1997;10:1-149.
10. Golan M, Weizman A, Apter A, Fainaru M. Am J Clin Nutr 1998;67:1130-5.
11. Barlow SE, Dietz WH. Pediatrics 1998;102:E29.-
1. Campbell K, Waters E, O’Meara S, et al. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
2. Simonetti D’Arca A, Tarsitani G, Cairella M, et al. Public Health 1986;100:166-73.
3. Robinson TN. JAMA 1999;282:1561-7.
4. Faith MS, Berman N, Heo M, et al. Pediatrics 2001;107:1043-8.
5. Donnelly JE, Jacobsen DJ, Whatley JE, et al. Obes Res 1996;4:229-43.
6. Gortmaker SL, Peterson K, Wiecha J, et al. Arch Pediatr Adolesc Med 1999;153:409-18.
7. Epstein LH, Paluch RA, Gordy CC, Dorn J. Arch Pediatr Adolesc Med 2000;154:220-6.
8. Epstein LH, Goldfield GS. Med Sci Sports Exerc 1999;31(suppl):S553-9.
9. Glenny AM, O’Meara S. CRD Report 1997;10:1-149.
10. Golan M, Weizman A, Apter A, Fainaru M. Am J Clin Nutr 1998;67:1130-5.
11. Barlow SE, Dietz WH. Pediatrics 1998;102:E29.-
Evidence-based answers from the Family Physicians Inquiries Network
What is the target for low-density lipoprotein cholesterol in patients with heart disease?
EVIDENCE-BASED ANSWER
Large published randomized controlled trials (RCTs) show that pravastatin and simvastatin are well-tolerated and reduce major coronary events such as death, myocardial infarction, and revascularization by about 25%. The Heart Protection Study suggested this benefit is noted even among individuals with pretreatment low-density lipoprotein (LDL) cholesterol of less than 100 mg/dL. Fluvastatin reduces major coronary events, but current studies are too small to prove reduced overall mortality. The best evidence to date suggests that most patients at significant risk for major coronary events should be given pravastatin or simvastatin 40 mg daily, without concern for the initial or follow-up LDL levels. (Grade of recommendation: A, based on large randomized trials.)
Evidence summary
The evidence is solid to support the use of HMG–CoA reductase inhibitors (statins) for patients with coronary artery disease (CAD). The Scandinavian Simvastatin Survival study used simvastatin 20 mg daily unless total cholesterol levels did not decrease to less than 200 mg/dL.1 The Long-Term Intervention with Pravastatin in Ischaemic Disease study randomized patients to pravastatin 40 mg daily or placebo, without titration.2 The intervention arm in the Cholesterol and Recurrent Events trial was also pravastatin 40 mg daily, with cholestyramine added if the LDL level remained higher than 175 mg/dL.3 The Lescol Intervention Prevention study randomized patients after angioplasty to fluvastatin 40 mg twice daily or placebo.4 The Heart Protection Study used simvastatin 40 mg daily, without titration.5 No RCTs have evaluated the clinical benefit of adding medications to adequate doses of statins to lower LDL to less than 175 mg/dL. See Table of major RCTs with clinical outcomes.
Subgroup analyses of earlier major RCTs had suggested that patients with CAD and low initial LDL levels (< 125 mg/dL) have little to gain from pravastatin.2,3 However, the Heart Protection Study enrolled 20,000 people with CAD or equivalent (diabetes, peripheral vascular disease, stroke, etc).5 The study demonstrated a reduction of major coronary events with simvastatin, with numbers needed to treat (NNT) of 19 (P < .0001); the NNT for reduction in all-cause mortality was 55 (P = .0003). The benefit of simvastatin was noted in virtually every predefined subgroup, including individuals older than 70 years, women, and patients without known CAD (but with CAD equivalents). Notably, no difference in benefit was found between patients with different pretreatment LDL levels. A significant reduction in major vascular events was noted even for the 3400 subjects with pretreatment LDL levels of less than 100 mg/dL (NNT = 22, P = .0006). A greater percentage reduction in LDL with medication did not predict better clinical outcomes.5
TABLE
LDL levels and relative risk of major coronary events after treatment
| Study, year | N | Intervention medication | LDL level, mg/dL | Relative risk of major coronary events | |
|---|---|---|---|---|---|
| Control group | Intervention group | ||||
| 4S,1 1994 | 4444 | Simvastatin 20 mg/d* | 190 | 122 | 0.66 (0.59–0.85) |
| CARE,3 1996 | 4159 | Pravastatin 40 mg/d | 139 | 97 | 0.76 (0.64–0.91) |
| LIPID,2 1998 | 9014 | Pravastatin 40 mg/d | 150 | 113 | 0.76 (0.68–0.85) |
| LIPS,4 2002 | 1677 | Fluvastatin 80 mg/d | 132 | 100 | 0.78 (0.64–0.95) |
| HPS,5 2002 | 20,536 | Simvastatin 40 mg/d | 127 | 89 | 0.76 (0.72–0.81) |
| *Increased to 40 mg/d if total cholesterol did not drop to less than 200 mg/dL. | |||||
| 4S, Scandinavian Simvastatin Survival Study; CARE, Cholesterol and Recurrent Events trial; HPS, Heart Protection Study; LDL, low-density lipoprotein; LIPID, Long-Term Intervention with Pravastatin in Ischaemic Disease study; LIPS, Lescol Intervention Prevention Study. | |||||
Recommendations from others
The National Cholesterol Education Project (NCEP) recommends that patients with CAD and an LDL of more than 130 mg/dL adopt therapeutic lifestyle changes and start LDL-lowering medication, usually a statin. For patients with LDL between 100 and 130 mg/dL, the NCEP recommends therapeutic lifestyle changes, with the option of adding a statin. For patients with LDL less than 100 mg/dL, maintenance of LDL control is recommended with therapeutic lifestyle changes. For patients with high initial LDL levels that stay above 100 mg/dL on statin therapy, the NCEP recommends that additional medications, such as nicotinic acid or fibrates, as well as intensive therapeutic lifestyle changes, be considered.6
Clinical Commentary by William Chavey, MD, at http://www.fpin.org.
1. The Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9.
2. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998;339:1349-57.
3. Sacks FM, Pfeffer MA, Moye LA, et al. N Engl J Med 1996;335:1001-9.
4. Serruys PW, De Feyter P, Macaya C, et al. JAMA 2002;287:3215-22.
5. Heart Protection Study Collaborative Group. Lancet 2002;360:7-22.
6. National Cholesterol Education Program. Adult Treatment Panel III Report. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.pdf. Accessed June 3, 2002.
EVIDENCE-BASED ANSWER
Large published randomized controlled trials (RCTs) show that pravastatin and simvastatin are well-tolerated and reduce major coronary events such as death, myocardial infarction, and revascularization by about 25%. The Heart Protection Study suggested this benefit is noted even among individuals with pretreatment low-density lipoprotein (LDL) cholesterol of less than 100 mg/dL. Fluvastatin reduces major coronary events, but current studies are too small to prove reduced overall mortality. The best evidence to date suggests that most patients at significant risk for major coronary events should be given pravastatin or simvastatin 40 mg daily, without concern for the initial or follow-up LDL levels. (Grade of recommendation: A, based on large randomized trials.)
Evidence summary
The evidence is solid to support the use of HMG–CoA reductase inhibitors (statins) for patients with coronary artery disease (CAD). The Scandinavian Simvastatin Survival study used simvastatin 20 mg daily unless total cholesterol levels did not decrease to less than 200 mg/dL.1 The Long-Term Intervention with Pravastatin in Ischaemic Disease study randomized patients to pravastatin 40 mg daily or placebo, without titration.2 The intervention arm in the Cholesterol and Recurrent Events trial was also pravastatin 40 mg daily, with cholestyramine added if the LDL level remained higher than 175 mg/dL.3 The Lescol Intervention Prevention study randomized patients after angioplasty to fluvastatin 40 mg twice daily or placebo.4 The Heart Protection Study used simvastatin 40 mg daily, without titration.5 No RCTs have evaluated the clinical benefit of adding medications to adequate doses of statins to lower LDL to less than 175 mg/dL. See Table of major RCTs with clinical outcomes.
Subgroup analyses of earlier major RCTs had suggested that patients with CAD and low initial LDL levels (< 125 mg/dL) have little to gain from pravastatin.2,3 However, the Heart Protection Study enrolled 20,000 people with CAD or equivalent (diabetes, peripheral vascular disease, stroke, etc).5 The study demonstrated a reduction of major coronary events with simvastatin, with numbers needed to treat (NNT) of 19 (P < .0001); the NNT for reduction in all-cause mortality was 55 (P = .0003). The benefit of simvastatin was noted in virtually every predefined subgroup, including individuals older than 70 years, women, and patients without known CAD (but with CAD equivalents). Notably, no difference in benefit was found between patients with different pretreatment LDL levels. A significant reduction in major vascular events was noted even for the 3400 subjects with pretreatment LDL levels of less than 100 mg/dL (NNT = 22, P = .0006). A greater percentage reduction in LDL with medication did not predict better clinical outcomes.5
TABLE
LDL levels and relative risk of major coronary events after treatment
| Study, year | N | Intervention medication | LDL level, mg/dL | Relative risk of major coronary events | |
|---|---|---|---|---|---|
| Control group | Intervention group | ||||
| 4S,1 1994 | 4444 | Simvastatin 20 mg/d* | 190 | 122 | 0.66 (0.59–0.85) |
| CARE,3 1996 | 4159 | Pravastatin 40 mg/d | 139 | 97 | 0.76 (0.64–0.91) |
| LIPID,2 1998 | 9014 | Pravastatin 40 mg/d | 150 | 113 | 0.76 (0.68–0.85) |
| LIPS,4 2002 | 1677 | Fluvastatin 80 mg/d | 132 | 100 | 0.78 (0.64–0.95) |
| HPS,5 2002 | 20,536 | Simvastatin 40 mg/d | 127 | 89 | 0.76 (0.72–0.81) |
| *Increased to 40 mg/d if total cholesterol did not drop to less than 200 mg/dL. | |||||
| 4S, Scandinavian Simvastatin Survival Study; CARE, Cholesterol and Recurrent Events trial; HPS, Heart Protection Study; LDL, low-density lipoprotein; LIPID, Long-Term Intervention with Pravastatin in Ischaemic Disease study; LIPS, Lescol Intervention Prevention Study. | |||||
Recommendations from others
The National Cholesterol Education Project (NCEP) recommends that patients with CAD and an LDL of more than 130 mg/dL adopt therapeutic lifestyle changes and start LDL-lowering medication, usually a statin. For patients with LDL between 100 and 130 mg/dL, the NCEP recommends therapeutic lifestyle changes, with the option of adding a statin. For patients with LDL less than 100 mg/dL, maintenance of LDL control is recommended with therapeutic lifestyle changes. For patients with high initial LDL levels that stay above 100 mg/dL on statin therapy, the NCEP recommends that additional medications, such as nicotinic acid or fibrates, as well as intensive therapeutic lifestyle changes, be considered.6
Clinical Commentary by William Chavey, MD, at http://www.fpin.org.
EVIDENCE-BASED ANSWER
Large published randomized controlled trials (RCTs) show that pravastatin and simvastatin are well-tolerated and reduce major coronary events such as death, myocardial infarction, and revascularization by about 25%. The Heart Protection Study suggested this benefit is noted even among individuals with pretreatment low-density lipoprotein (LDL) cholesterol of less than 100 mg/dL. Fluvastatin reduces major coronary events, but current studies are too small to prove reduced overall mortality. The best evidence to date suggests that most patients at significant risk for major coronary events should be given pravastatin or simvastatin 40 mg daily, without concern for the initial or follow-up LDL levels. (Grade of recommendation: A, based on large randomized trials.)
Evidence summary
The evidence is solid to support the use of HMG–CoA reductase inhibitors (statins) for patients with coronary artery disease (CAD). The Scandinavian Simvastatin Survival study used simvastatin 20 mg daily unless total cholesterol levels did not decrease to less than 200 mg/dL.1 The Long-Term Intervention with Pravastatin in Ischaemic Disease study randomized patients to pravastatin 40 mg daily or placebo, without titration.2 The intervention arm in the Cholesterol and Recurrent Events trial was also pravastatin 40 mg daily, with cholestyramine added if the LDL level remained higher than 175 mg/dL.3 The Lescol Intervention Prevention study randomized patients after angioplasty to fluvastatin 40 mg twice daily or placebo.4 The Heart Protection Study used simvastatin 40 mg daily, without titration.5 No RCTs have evaluated the clinical benefit of adding medications to adequate doses of statins to lower LDL to less than 175 mg/dL. See Table of major RCTs with clinical outcomes.
Subgroup analyses of earlier major RCTs had suggested that patients with CAD and low initial LDL levels (< 125 mg/dL) have little to gain from pravastatin.2,3 However, the Heart Protection Study enrolled 20,000 people with CAD or equivalent (diabetes, peripheral vascular disease, stroke, etc).5 The study demonstrated a reduction of major coronary events with simvastatin, with numbers needed to treat (NNT) of 19 (P < .0001); the NNT for reduction in all-cause mortality was 55 (P = .0003). The benefit of simvastatin was noted in virtually every predefined subgroup, including individuals older than 70 years, women, and patients without known CAD (but with CAD equivalents). Notably, no difference in benefit was found between patients with different pretreatment LDL levels. A significant reduction in major vascular events was noted even for the 3400 subjects with pretreatment LDL levels of less than 100 mg/dL (NNT = 22, P = .0006). A greater percentage reduction in LDL with medication did not predict better clinical outcomes.5
TABLE
LDL levels and relative risk of major coronary events after treatment
| Study, year | N | Intervention medication | LDL level, mg/dL | Relative risk of major coronary events | |
|---|---|---|---|---|---|
| Control group | Intervention group | ||||
| 4S,1 1994 | 4444 | Simvastatin 20 mg/d* | 190 | 122 | 0.66 (0.59–0.85) |
| CARE,3 1996 | 4159 | Pravastatin 40 mg/d | 139 | 97 | 0.76 (0.64–0.91) |
| LIPID,2 1998 | 9014 | Pravastatin 40 mg/d | 150 | 113 | 0.76 (0.68–0.85) |
| LIPS,4 2002 | 1677 | Fluvastatin 80 mg/d | 132 | 100 | 0.78 (0.64–0.95) |
| HPS,5 2002 | 20,536 | Simvastatin 40 mg/d | 127 | 89 | 0.76 (0.72–0.81) |
| *Increased to 40 mg/d if total cholesterol did not drop to less than 200 mg/dL. | |||||
| 4S, Scandinavian Simvastatin Survival Study; CARE, Cholesterol and Recurrent Events trial; HPS, Heart Protection Study; LDL, low-density lipoprotein; LIPID, Long-Term Intervention with Pravastatin in Ischaemic Disease study; LIPS, Lescol Intervention Prevention Study. | |||||
Recommendations from others
The National Cholesterol Education Project (NCEP) recommends that patients with CAD and an LDL of more than 130 mg/dL adopt therapeutic lifestyle changes and start LDL-lowering medication, usually a statin. For patients with LDL between 100 and 130 mg/dL, the NCEP recommends therapeutic lifestyle changes, with the option of adding a statin. For patients with LDL less than 100 mg/dL, maintenance of LDL control is recommended with therapeutic lifestyle changes. For patients with high initial LDL levels that stay above 100 mg/dL on statin therapy, the NCEP recommends that additional medications, such as nicotinic acid or fibrates, as well as intensive therapeutic lifestyle changes, be considered.6
Clinical Commentary by William Chavey, MD, at http://www.fpin.org.
1. The Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9.
2. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998;339:1349-57.
3. Sacks FM, Pfeffer MA, Moye LA, et al. N Engl J Med 1996;335:1001-9.
4. Serruys PW, De Feyter P, Macaya C, et al. JAMA 2002;287:3215-22.
5. Heart Protection Study Collaborative Group. Lancet 2002;360:7-22.
6. National Cholesterol Education Program. Adult Treatment Panel III Report. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.pdf. Accessed June 3, 2002.
1. The Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9.
2. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998;339:1349-57.
3. Sacks FM, Pfeffer MA, Moye LA, et al. N Engl J Med 1996;335:1001-9.
4. Serruys PW, De Feyter P, Macaya C, et al. JAMA 2002;287:3215-22.
5. Heart Protection Study Collaborative Group. Lancet 2002;360:7-22.
6. National Cholesterol Education Program. Adult Treatment Panel III Report. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.pdf. Accessed June 3, 2002.
Evidence-based answers from the Family Physicians Inquiries Network