User login
Imaging for autonomic dysfunction
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
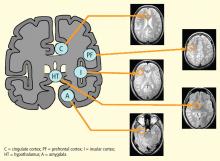
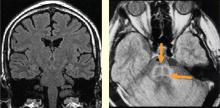
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
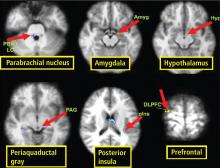
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
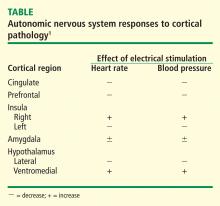
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
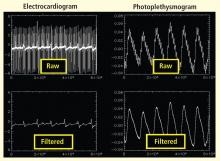
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10
FUNCTIONAL BRAIN IMAGING IN GENERAL
Direct visualization of heart-brain interactions is the goal when assessing ANS function. Positron emission tomography (PET) produces quantitative images, but spatial and temporal resolutions are vastly superior with fMRI.11 Further, radiation exposure is low with fMRI, allowing for safe repeat imaging.
Ogawa et al12 first demonstrated that in vivo images of brain microvasculature are affected by blood oxygen level, and that blood oxygenation reduced vascular signal loss. Therefore, blood oxygenation level–dependent (BOLD) contrast added to MRI could complement PET-like measurements in the study of regional brain activity.
Bilateral finger tapping with intermittent periods of rest is associated with a pattern of increasing and decreasing intensity of fMRI signals in involved brain regions that reflect the periods of activity and rest. This technique has been used to locate brain voxels with similar patterns of activity, enabling the creation of familiar color brain mapping. A challenge posed by autonomic fMRI in such brain mapping is that fMRI is susceptible to artifacts (Figure 3). For example, a movement of the head as little as 1 mm inside the MRI scanner—a distance comparable to the size of autonomic structures—can produce a motion artifact (false activation of brain regions) that can affect statistical significance. In addition, many ANS regions of the brain are near osseous structures (for example the brainstem and skull base) that cause signal distortion and loss.
REQUIREMENTS FOR AUTONOMIC fMRI
The tasks chosen to visualize brain control of autonomic function must naturally elicit an autonomic response. The difficulty is that untrained persons have little or no volitional control over autonomic functions, so the task and its analysis must be designed carefully and be MRI-compatible. Any motion will degrade the image; further, the capacity for the MRI environment to corrupt the measurements can limit the potential tasks for measurement.
Possible stimuli for eliciting a sympathetic response include pain, fear, anticipation, anxiety, concentration or memory, cold pressor, Stroop test, breathing tests, and maximal hand grip. Examples of parasympathetic stimuli are the Valsalva maneuver and paced breathing. The responses to stimuli (ie, heart rate, heart rate variability, blood pressure, galvanic skin response, papillary response) must be monitored to compare the data obtained from fMRI. MRI-compatible equipment is now available for measuring many of these responses.
Identifying areas activated during tasks
Functional neuroimaging with PET and fMRI has shown consistently that the anterior cingulate is activated during multiple tasks designed to elicit an autonomic response (gambling anticipation, emotional response to faces, Stroop test).11
In a study designed to test autonomic interoceptive awareness, subjects underwent fMRI while they were asked to judge the timing of their heartbeats to auditory tones that were either synchronized with their heartbeat or delayed by 500 msec.13 Areas of enhanced activity during the task were the right insular cortex, anterior cingulate, parietal lobes, and operculum.
Characterizing brainstem sites
It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses. These regions are small and motion artifacts are common because of brainstem movement with the cardiac pulse. With fMRI, Topolovec et al14 were able to characterize brainstem sites involved in autonomic control, demonstrating activation of the nucleus of the solitary tract and parabrachial nucleus.
A review of four fMRI studies of stressor-evoked blood pressure reactivity demonstrated activation in corticolimbic areas, including the cingulate cortex, insula, amygdala, and cortical and subcortical areas that are involved in hemodynamic and metabolic support for stress-related behavioral responses.16
FUNCTIONAL BRAIN IMAGING IN DISEASE STATES
There are few studies of functional brain imaging in patients with disease because of the challenges involved. The studies are difficult to perform on sick patients because of the unfriendly MRI environment, with struct requirements for attention and participation. Furthermore, autonomic responses may be blunted, making physiologic comparisons difficult. In addition, there is evidence that BOLD may be intrinsically impaired in disease states. Unlike fMRI studies to locate brain regions involved in simple tasks such as finger tapping, which can be performed in a single subject, detecting changes in autonomic responses in disease states requires averaging over studies of multiple patients.
Woo et al17 used fMRI to compare brain regions of activation in six patients with heart failure and 16 controls upon a forehead cold pressor challenge. Increases in heart rate were measured in the patients with heart failure with application of the cold stimulus. Larger neural fMRI signal responses in patients with heart failure were observed in 14 brain regions, whereas reduced fMRI activity was observed in 15 other brain regions in the heart failure patients. Based on the results, the investigators suggested that heart failure may be associated with altered sympathetic and parasympathetic activity, and that these dysfunctions might contribute to the progression of heart failure.
Gianaros et al18 found fMRI evidence for a correlation between carotid artery intima-media thickness, a surrogate measure for carotid artery or coronary artery disease, and altered ANS reaction to fear using a fearful faces paradigm.
CONCLUSION
Functional MRI of heart-brain interactions has strong potential for normal subjects, in whom the BOLD effect is small, within the limits of motion and susceptibility artifacts. Typically, such applications require averaging results over multiple subjects. Its potential utility in disease states is less significant because of the additional limitations of MRI with sick patients (the MRI environment, blunting of autonomic response in disease, possible impairment of BOLD), but continued investigation is warranted.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.
- Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51:725–737.
- Kodounis A, Stamboulis E, Constantinidis TS, Liolios A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112:403–408.
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999; 246:578–586.
- Kulcu DG, Akbas B, Citci B, Cihangiroglu M. Autonomic dysreflexia in a man with multiple sclerosis. J Spinal Cord Med 2009; 32:198–203.
- Abboud H, Berroir S, Labreuche J, Orjuele K, Amarenco O. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59:691–699.
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30:1301–1311.
- Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol 2003; 50:30–38.
- Lassman AB, Mayer SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol 2005; 62:1286–1288.
- Deluca C, Tinazzi M, Bovi P, Rizzuto N, Moretto G. Limb ataxia and proximal intracranial territory brain infarcts: clinical and topographical correlations. J Neurol Neurosurg Psychiatry 2007; 78:832–835.
- Gómez-Esteban JC, Berganzo K, Tijero B, Barcena J, Zarranz JJ. Orthostatic hypotension associated with an epidermoid tumor of the IV ventricle. J Neurol 2009; 256:1357–1359.
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872.
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 2004; 101:6333–6334.
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol 2004; 471:446–461.
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 2008; 42:169–177.
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009; 47:922–936.
- Woo MA, Macey PM, Keens PT, et al Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11:437–446.
- Gianaros PJ, Hariri AR, Sheu LK, et al Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psych 2009; 65:943–950.