User login
Inverted Appendix in a Patient With Weakness and Occult Bleeding
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
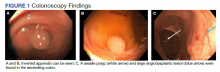
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
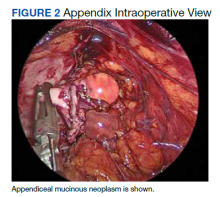
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
Constipation and Postprandial Pain in a Patient With Shortness of Breath
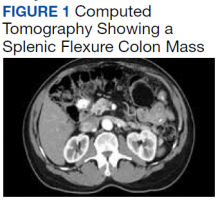
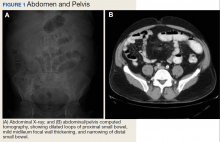
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
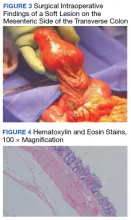
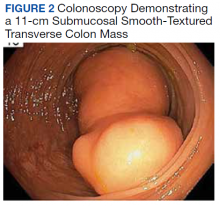
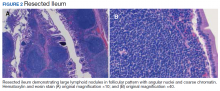
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
Left-Sided Amyand Hernia: Case Report and Review of the Literature
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
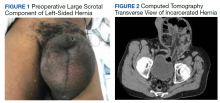
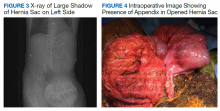
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Small Bowel Obstruction in a Surgically Naïve Abdomen
A 53-year-old male veteran with a history of heavy tobacco and alcohol use presented with abdominal pain, emesis, and no bowel movements for 2 days. He had no history of surgical procedures, malignancies, diverticulitis, inflammatory bowel disease, traveling abroad, parasitic infections, tuberculosis exposure, or hospital admissions for abdominal pain. He reported experiencing no flushing, diarrhea, or cardiac symptoms. His medical history included hypertension, depression, and osteoarthritis. His vital signs were within normal limits.
A physical examination revealed a distended abdomen with mild tenderness. He had no inguinal or ventral hernias. He also had no abnormal skin lesions. A rectal examination did not reveal any masses or blood. His laboratory values were normal. X-ray and computed tomography (CT) scan revealed dilated loops of proximal small bowel, mild wall thickening in a segment of the midileum, and narrowing of the distal small bowel suggestive of a partial small bowel obstruction (Figure 1). A 1-cm nonspecific omental nodule also was seen on the CT scan, but no enlarged lymph nodes or mesenteric calcifications were seen. There was no thickening of the terminal ileum.
The patient underwent an exploratory laparotomy, which revealed no adhesions. In the midileum there was an area of thickened bowel with some nodularity associated with the thickness, but no discrete mass. In the mesentery there were multiple hard, white, calcified nodules, with the majority clustered near the thickened ileal segment. There also was a 1-cm hard, peritoneal mass on the anterior abdominal wall. The segment of thickened ileum, the adjacent mesentery, and the peritoneal nodule were resected.
Pathologic examination of the resected tissue showed immunohistochemical stains that were positive for CD79a, CD10, and BCL-2 and negative for CD23, CD5, and CD3. Nineteen mesenteric lymph nodes were negative for malignancy. The postoperative staging positron emission tomography (PET) scan did not reveal any fluorodeoxyglucose avid masses anywhere else, and bone marrow biopsy showed no infiltration.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the pathologic examination of the resected tissue and immunohistochemical stains, this patient was diagnosed with malignant non-Hodgkin B-cell lymphoma, follicular type, grade 1. PET scan and bone marrow biopsy revealed no other lesions, making this a primary lymphoma of the small intestine. The resected tissue showed negative margins and negative lymph nodes, indicating the full extent of the patient’s tumor was removed. He then underwent nasogastric tube decompression and IV fluid resuscitation. Two days later, he had a large bowel movement, and his abdominal pain resolved. He was provided the treatment options of observation only, radiation therapy, or rituximab treatment. Based on the high risk of enteritis following radiation therapy, the patient elected for observation only, with a repeat scan in 6 months. He also was counseled on alcohol and tobacco cessation. At the 6-month oncology follow-up, the patient showed no evidence of disease recurrence.
Discussion
Small bowel obstruction accounts for about 350,000 hospitalizations annually in the US.1 The incidence is equal in men and women and can present at any age.2,3 Patients typically present nonspecifically, with intermittent, colicky abdominal pain, nausea, vomiting, and constipation.2 A physical examination may reveal abdominal distention, rigidity, and hypoactive or absent bowel sounds.1 The 2 most common etiologies of small bowel obstruction are adhesions from prior abdominal surgery (65%) and incarcerated inguinal hernias (10%).1 However, in a patient presenting with a small bowel obstruction in a surgically naïve abdomen with no hernias, a more detailed history covering current malignancies, past hospital admissions for abdominal pains, pelvic inflammatory disease, diverticulitis, inflammatory bowel disease, and risks for parasite infection must be taken. The differential should include intraluminal causes, including small bowel malignancy, which accounts for 5% of small bowel obstructions,1 as well as extraluminal causes, including adhesions from diverticulitis, Meckel diverticulum, Ladd bands, and undiagnosed prior appendicitis.
To provide a tissue diagnosis and definitive treatment, surgical exploration was needed for this patient. Exploratory laparotomy revealed an area of thickened ileum and calcified nodules in its mesentery. Pathologic examination of the resected tissue revealed large lymphoid nodules in a follicular pattern with coarse chromatin (Figure 2). Taken together with the immunohistochemical stains, this was consistent with malignant B-cell non-Hodgkin lymphoma, follicular type, grade 1.
Small bowel malignancy accounts for > 5% of all gastrointestinal tumors.4 Of these, small bowel neuroendocrine tumors are the most common, followed by adenocarcinomas, lymphomas, and stromal tumors.4 Primary follicular lymphoma (PFL) is a B-cell non-Hodgkin lymphoma, and comprises between 3.8% and 11% of gastrointestinal lymphomas, commonly in the duodenum and terminal ileum.5
PFL typically occurs in middle-aged females and can be difficult to diagnose, as most patients are asymptomatic or present with unspecified abdominal pain. Many are diagnosed incidentally when endoscopy biopsies are performed for other reasons.4,5 Histologically, PFL is composed of a mixed population of small (centrocytes) and large (centroblasts) lymphoid cells, with higher proportions of centroblasts corresponding to a higher grade lymphoma.6 The classic immunophenotype of PFL shows coexpression of CD79a (or CD20), CD10, and BCL-2; however, in rare cases, low-grade PFL may stain negative for BCL-2 and have diminished staining for CD10 in interfollicular areas.7
PFL generally carries a favorable prognosis. Most patients achieving complete disease regression or stable disease following treatment and a low recurrence rate. Treatment can include surgical resection, radiation, rituximab therapy, chemotherapy, or observation.8 Patient also should be counseled in alcohol and tobacco cessation to reduce recurrence risk.
Other small bowel malignancies may present as small bowel obstructions as well. Neuroendocrine tumors and adenocarcinomas are both more common than small bowel lymphomas and can present as small bowel obstruction. However, neuroendocrine tumors are derived from serotonin-expressing enterochromaffin cells of the midgut and often present with classic carcinoid syndrome symptoms, including diarrhea, flushing, and right heart fibrosis, which the patient lacked.9 Immunohistology of small bowel adenocarcinoma often shows expression of MUC1 or MUC5AC with tumor markers CEA and CA 19-9.10
Primary intestinal melanoma, another small bowel malignancy, is extremely rare. More commonly, the etiology of intestinal melanoma is cutaneous melanoma that metastasizes to the gastrointestinal tract.11 This patient had no skin lesions to suggest metastatic melanoma. With intestinal melanoma, immunohistochemical evaluation may show S-100, the most sensitive marker for melanoma, or HMB-45, MART-1/Melan-A, tyrosinase, and MITF.12
Conclusion
This case is notable because it highlights the importance of examining the cause of small bowel obstruction in a surgically naïve abdomen, as exploration led to the discovery and curative treatment of a primary intestinal malignancy. It also underscores the nonspecific presentation that PFLs of the small intestine can have and the importance of understanding the different histopathology and immunohistochemical profiles of small bowel malignancies.
1. Rami Reddy SR, Cappell MS. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr Gastroenterol Rep. 2017;19(6):28.
2. Smith DA, Nehring SM. Bowel obstruction. https://www.ncbi.nlm.nih.gov/books/NBK441975. Updated November 12, 2019. Accessed February 6, 2020.
3. Popoola D, Lou MA, Mansour AY, Sims EH. Small bowel obstruction: review of nine years of experience. J Natl Med Assoc. 1984;76(11):1089-1094.
4. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63-71.
5. Freedman AS. Clinical presentation and diagnosis of primary gastrointestinal lymphomas. https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-primary-gastrointestinal-lymphomas. Updated March 26, 2019. Accessed February 6, 2020.
6. Moy BT, Wilmot J, Ballesteros E, Forouhar F, Vaziri H. Primary follicular lymphoma of the gastrointestinal tract: casereport and review. J Gastrointest Cancer. 2016;47(3):255-263.
7. Choi SM, Betz BL, Perry AM. Follicular lymphoma diagnostic caveats and updates. Arch Pathol Lab Med. 2018;142(11):1330-1340.
8. Schmatz AI, Streubel B, Kretschmer-Chott E, et al. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29(11):1445-1451.
9. Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med. 2015;139(6):750-756.
10. Chang H-K, Yu E, Kim J, et al; Korean Small Intestinal Cancer Study Group. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol. 2010;41(8):1087-1096.
11. Lens M, Bataille V, Krivokapic Z. Melanoma of the small intestine. Lancet Oncol. 2009;10(5):516-521.
12. Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35(5):433-444.
A 53-year-old male veteran with a history of heavy tobacco and alcohol use presented with abdominal pain, emesis, and no bowel movements for 2 days. He had no history of surgical procedures, malignancies, diverticulitis, inflammatory bowel disease, traveling abroad, parasitic infections, tuberculosis exposure, or hospital admissions for abdominal pain. He reported experiencing no flushing, diarrhea, or cardiac symptoms. His medical history included hypertension, depression, and osteoarthritis. His vital signs were within normal limits.
A physical examination revealed a distended abdomen with mild tenderness. He had no inguinal or ventral hernias. He also had no abnormal skin lesions. A rectal examination did not reveal any masses or blood. His laboratory values were normal. X-ray and computed tomography (CT) scan revealed dilated loops of proximal small bowel, mild wall thickening in a segment of the midileum, and narrowing of the distal small bowel suggestive of a partial small bowel obstruction (Figure 1). A 1-cm nonspecific omental nodule also was seen on the CT scan, but no enlarged lymph nodes or mesenteric calcifications were seen. There was no thickening of the terminal ileum.
The patient underwent an exploratory laparotomy, which revealed no adhesions. In the midileum there was an area of thickened bowel with some nodularity associated with the thickness, but no discrete mass. In the mesentery there were multiple hard, white, calcified nodules, with the majority clustered near the thickened ileal segment. There also was a 1-cm hard, peritoneal mass on the anterior abdominal wall. The segment of thickened ileum, the adjacent mesentery, and the peritoneal nodule were resected.
Pathologic examination of the resected tissue showed immunohistochemical stains that were positive for CD79a, CD10, and BCL-2 and negative for CD23, CD5, and CD3. Nineteen mesenteric lymph nodes were negative for malignancy. The postoperative staging positron emission tomography (PET) scan did not reveal any fluorodeoxyglucose avid masses anywhere else, and bone marrow biopsy showed no infiltration.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the pathologic examination of the resected tissue and immunohistochemical stains, this patient was diagnosed with malignant non-Hodgkin B-cell lymphoma, follicular type, grade 1. PET scan and bone marrow biopsy revealed no other lesions, making this a primary lymphoma of the small intestine. The resected tissue showed negative margins and negative lymph nodes, indicating the full extent of the patient’s tumor was removed. He then underwent nasogastric tube decompression and IV fluid resuscitation. Two days later, he had a large bowel movement, and his abdominal pain resolved. He was provided the treatment options of observation only, radiation therapy, or rituximab treatment. Based on the high risk of enteritis following radiation therapy, the patient elected for observation only, with a repeat scan in 6 months. He also was counseled on alcohol and tobacco cessation. At the 6-month oncology follow-up, the patient showed no evidence of disease recurrence.
Discussion
Small bowel obstruction accounts for about 350,000 hospitalizations annually in the US.1 The incidence is equal in men and women and can present at any age.2,3 Patients typically present nonspecifically, with intermittent, colicky abdominal pain, nausea, vomiting, and constipation.2 A physical examination may reveal abdominal distention, rigidity, and hypoactive or absent bowel sounds.1 The 2 most common etiologies of small bowel obstruction are adhesions from prior abdominal surgery (65%) and incarcerated inguinal hernias (10%).1 However, in a patient presenting with a small bowel obstruction in a surgically naïve abdomen with no hernias, a more detailed history covering current malignancies, past hospital admissions for abdominal pains, pelvic inflammatory disease, diverticulitis, inflammatory bowel disease, and risks for parasite infection must be taken. The differential should include intraluminal causes, including small bowel malignancy, which accounts for 5% of small bowel obstructions,1 as well as extraluminal causes, including adhesions from diverticulitis, Meckel diverticulum, Ladd bands, and undiagnosed prior appendicitis.
To provide a tissue diagnosis and definitive treatment, surgical exploration was needed for this patient. Exploratory laparotomy revealed an area of thickened ileum and calcified nodules in its mesentery. Pathologic examination of the resected tissue revealed large lymphoid nodules in a follicular pattern with coarse chromatin (Figure 2). Taken together with the immunohistochemical stains, this was consistent with malignant B-cell non-Hodgkin lymphoma, follicular type, grade 1.
Small bowel malignancy accounts for > 5% of all gastrointestinal tumors.4 Of these, small bowel neuroendocrine tumors are the most common, followed by adenocarcinomas, lymphomas, and stromal tumors.4 Primary follicular lymphoma (PFL) is a B-cell non-Hodgkin lymphoma, and comprises between 3.8% and 11% of gastrointestinal lymphomas, commonly in the duodenum and terminal ileum.5
PFL typically occurs in middle-aged females and can be difficult to diagnose, as most patients are asymptomatic or present with unspecified abdominal pain. Many are diagnosed incidentally when endoscopy biopsies are performed for other reasons.4,5 Histologically, PFL is composed of a mixed population of small (centrocytes) and large (centroblasts) lymphoid cells, with higher proportions of centroblasts corresponding to a higher grade lymphoma.6 The classic immunophenotype of PFL shows coexpression of CD79a (or CD20), CD10, and BCL-2; however, in rare cases, low-grade PFL may stain negative for BCL-2 and have diminished staining for CD10 in interfollicular areas.7
PFL generally carries a favorable prognosis. Most patients achieving complete disease regression or stable disease following treatment and a low recurrence rate. Treatment can include surgical resection, radiation, rituximab therapy, chemotherapy, or observation.8 Patient also should be counseled in alcohol and tobacco cessation to reduce recurrence risk.
Other small bowel malignancies may present as small bowel obstructions as well. Neuroendocrine tumors and adenocarcinomas are both more common than small bowel lymphomas and can present as small bowel obstruction. However, neuroendocrine tumors are derived from serotonin-expressing enterochromaffin cells of the midgut and often present with classic carcinoid syndrome symptoms, including diarrhea, flushing, and right heart fibrosis, which the patient lacked.9 Immunohistology of small bowel adenocarcinoma often shows expression of MUC1 or MUC5AC with tumor markers CEA and CA 19-9.10
Primary intestinal melanoma, another small bowel malignancy, is extremely rare. More commonly, the etiology of intestinal melanoma is cutaneous melanoma that metastasizes to the gastrointestinal tract.11 This patient had no skin lesions to suggest metastatic melanoma. With intestinal melanoma, immunohistochemical evaluation may show S-100, the most sensitive marker for melanoma, or HMB-45, MART-1/Melan-A, tyrosinase, and MITF.12
Conclusion
This case is notable because it highlights the importance of examining the cause of small bowel obstruction in a surgically naïve abdomen, as exploration led to the discovery and curative treatment of a primary intestinal malignancy. It also underscores the nonspecific presentation that PFLs of the small intestine can have and the importance of understanding the different histopathology and immunohistochemical profiles of small bowel malignancies.
A 53-year-old male veteran with a history of heavy tobacco and alcohol use presented with abdominal pain, emesis, and no bowel movements for 2 days. He had no history of surgical procedures, malignancies, diverticulitis, inflammatory bowel disease, traveling abroad, parasitic infections, tuberculosis exposure, or hospital admissions for abdominal pain. He reported experiencing no flushing, diarrhea, or cardiac symptoms. His medical history included hypertension, depression, and osteoarthritis. His vital signs were within normal limits.
A physical examination revealed a distended abdomen with mild tenderness. He had no inguinal or ventral hernias. He also had no abnormal skin lesions. A rectal examination did not reveal any masses or blood. His laboratory values were normal. X-ray and computed tomography (CT) scan revealed dilated loops of proximal small bowel, mild wall thickening in a segment of the midileum, and narrowing of the distal small bowel suggestive of a partial small bowel obstruction (Figure 1). A 1-cm nonspecific omental nodule also was seen on the CT scan, but no enlarged lymph nodes or mesenteric calcifications were seen. There was no thickening of the terminal ileum.
The patient underwent an exploratory laparotomy, which revealed no adhesions. In the midileum there was an area of thickened bowel with some nodularity associated with the thickness, but no discrete mass. In the mesentery there were multiple hard, white, calcified nodules, with the majority clustered near the thickened ileal segment. There also was a 1-cm hard, peritoneal mass on the anterior abdominal wall. The segment of thickened ileum, the adjacent mesentery, and the peritoneal nodule were resected.
Pathologic examination of the resected tissue showed immunohistochemical stains that were positive for CD79a, CD10, and BCL-2 and negative for CD23, CD5, and CD3. Nineteen mesenteric lymph nodes were negative for malignancy. The postoperative staging positron emission tomography (PET) scan did not reveal any fluorodeoxyglucose avid masses anywhere else, and bone marrow biopsy showed no infiltration.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the pathologic examination of the resected tissue and immunohistochemical stains, this patient was diagnosed with malignant non-Hodgkin B-cell lymphoma, follicular type, grade 1. PET scan and bone marrow biopsy revealed no other lesions, making this a primary lymphoma of the small intestine. The resected tissue showed negative margins and negative lymph nodes, indicating the full extent of the patient’s tumor was removed. He then underwent nasogastric tube decompression and IV fluid resuscitation. Two days later, he had a large bowel movement, and his abdominal pain resolved. He was provided the treatment options of observation only, radiation therapy, or rituximab treatment. Based on the high risk of enteritis following radiation therapy, the patient elected for observation only, with a repeat scan in 6 months. He also was counseled on alcohol and tobacco cessation. At the 6-month oncology follow-up, the patient showed no evidence of disease recurrence.
Discussion
Small bowel obstruction accounts for about 350,000 hospitalizations annually in the US.1 The incidence is equal in men and women and can present at any age.2,3 Patients typically present nonspecifically, with intermittent, colicky abdominal pain, nausea, vomiting, and constipation.2 A physical examination may reveal abdominal distention, rigidity, and hypoactive or absent bowel sounds.1 The 2 most common etiologies of small bowel obstruction are adhesions from prior abdominal surgery (65%) and incarcerated inguinal hernias (10%).1 However, in a patient presenting with a small bowel obstruction in a surgically naïve abdomen with no hernias, a more detailed history covering current malignancies, past hospital admissions for abdominal pains, pelvic inflammatory disease, diverticulitis, inflammatory bowel disease, and risks for parasite infection must be taken. The differential should include intraluminal causes, including small bowel malignancy, which accounts for 5% of small bowel obstructions,1 as well as extraluminal causes, including adhesions from diverticulitis, Meckel diverticulum, Ladd bands, and undiagnosed prior appendicitis.
To provide a tissue diagnosis and definitive treatment, surgical exploration was needed for this patient. Exploratory laparotomy revealed an area of thickened ileum and calcified nodules in its mesentery. Pathologic examination of the resected tissue revealed large lymphoid nodules in a follicular pattern with coarse chromatin (Figure 2). Taken together with the immunohistochemical stains, this was consistent with malignant B-cell non-Hodgkin lymphoma, follicular type, grade 1.
Small bowel malignancy accounts for > 5% of all gastrointestinal tumors.4 Of these, small bowel neuroendocrine tumors are the most common, followed by adenocarcinomas, lymphomas, and stromal tumors.4 Primary follicular lymphoma (PFL) is a B-cell non-Hodgkin lymphoma, and comprises between 3.8% and 11% of gastrointestinal lymphomas, commonly in the duodenum and terminal ileum.5
PFL typically occurs in middle-aged females and can be difficult to diagnose, as most patients are asymptomatic or present with unspecified abdominal pain. Many are diagnosed incidentally when endoscopy biopsies are performed for other reasons.4,5 Histologically, PFL is composed of a mixed population of small (centrocytes) and large (centroblasts) lymphoid cells, with higher proportions of centroblasts corresponding to a higher grade lymphoma.6 The classic immunophenotype of PFL shows coexpression of CD79a (or CD20), CD10, and BCL-2; however, in rare cases, low-grade PFL may stain negative for BCL-2 and have diminished staining for CD10 in interfollicular areas.7
PFL generally carries a favorable prognosis. Most patients achieving complete disease regression or stable disease following treatment and a low recurrence rate. Treatment can include surgical resection, radiation, rituximab therapy, chemotherapy, or observation.8 Patient also should be counseled in alcohol and tobacco cessation to reduce recurrence risk.
Other small bowel malignancies may present as small bowel obstructions as well. Neuroendocrine tumors and adenocarcinomas are both more common than small bowel lymphomas and can present as small bowel obstruction. However, neuroendocrine tumors are derived from serotonin-expressing enterochromaffin cells of the midgut and often present with classic carcinoid syndrome symptoms, including diarrhea, flushing, and right heart fibrosis, which the patient lacked.9 Immunohistology of small bowel adenocarcinoma often shows expression of MUC1 or MUC5AC with tumor markers CEA and CA 19-9.10
Primary intestinal melanoma, another small bowel malignancy, is extremely rare. More commonly, the etiology of intestinal melanoma is cutaneous melanoma that metastasizes to the gastrointestinal tract.11 This patient had no skin lesions to suggest metastatic melanoma. With intestinal melanoma, immunohistochemical evaluation may show S-100, the most sensitive marker for melanoma, or HMB-45, MART-1/Melan-A, tyrosinase, and MITF.12
Conclusion
This case is notable because it highlights the importance of examining the cause of small bowel obstruction in a surgically naïve abdomen, as exploration led to the discovery and curative treatment of a primary intestinal malignancy. It also underscores the nonspecific presentation that PFLs of the small intestine can have and the importance of understanding the different histopathology and immunohistochemical profiles of small bowel malignancies.
1. Rami Reddy SR, Cappell MS. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr Gastroenterol Rep. 2017;19(6):28.
2. Smith DA, Nehring SM. Bowel obstruction. https://www.ncbi.nlm.nih.gov/books/NBK441975. Updated November 12, 2019. Accessed February 6, 2020.
3. Popoola D, Lou MA, Mansour AY, Sims EH. Small bowel obstruction: review of nine years of experience. J Natl Med Assoc. 1984;76(11):1089-1094.
4. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63-71.
5. Freedman AS. Clinical presentation and diagnosis of primary gastrointestinal lymphomas. https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-primary-gastrointestinal-lymphomas. Updated March 26, 2019. Accessed February 6, 2020.
6. Moy BT, Wilmot J, Ballesteros E, Forouhar F, Vaziri H. Primary follicular lymphoma of the gastrointestinal tract: casereport and review. J Gastrointest Cancer. 2016;47(3):255-263.
7. Choi SM, Betz BL, Perry AM. Follicular lymphoma diagnostic caveats and updates. Arch Pathol Lab Med. 2018;142(11):1330-1340.
8. Schmatz AI, Streubel B, Kretschmer-Chott E, et al. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29(11):1445-1451.
9. Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med. 2015;139(6):750-756.
10. Chang H-K, Yu E, Kim J, et al; Korean Small Intestinal Cancer Study Group. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol. 2010;41(8):1087-1096.
11. Lens M, Bataille V, Krivokapic Z. Melanoma of the small intestine. Lancet Oncol. 2009;10(5):516-521.
12. Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35(5):433-444.
1. Rami Reddy SR, Cappell MS. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr Gastroenterol Rep. 2017;19(6):28.
2. Smith DA, Nehring SM. Bowel obstruction. https://www.ncbi.nlm.nih.gov/books/NBK441975. Updated November 12, 2019. Accessed February 6, 2020.
3. Popoola D, Lou MA, Mansour AY, Sims EH. Small bowel obstruction: review of nine years of experience. J Natl Med Assoc. 1984;76(11):1089-1094.
4. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63-71.
5. Freedman AS. Clinical presentation and diagnosis of primary gastrointestinal lymphomas. https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-primary-gastrointestinal-lymphomas. Updated March 26, 2019. Accessed February 6, 2020.
6. Moy BT, Wilmot J, Ballesteros E, Forouhar F, Vaziri H. Primary follicular lymphoma of the gastrointestinal tract: casereport and review. J Gastrointest Cancer. 2016;47(3):255-263.
7. Choi SM, Betz BL, Perry AM. Follicular lymphoma diagnostic caveats and updates. Arch Pathol Lab Med. 2018;142(11):1330-1340.
8. Schmatz AI, Streubel B, Kretschmer-Chott E, et al. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29(11):1445-1451.
9. Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med. 2015;139(6):750-756.
10. Chang H-K, Yu E, Kim J, et al; Korean Small Intestinal Cancer Study Group. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol. 2010;41(8):1087-1096.
11. Lens M, Bataille V, Krivokapic Z. Melanoma of the small intestine. Lancet Oncol. 2009;10(5):516-521.
12. Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35(5):433-444.