User login
Breastfeeding by patients with serious mental illness: An ethical approach
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21
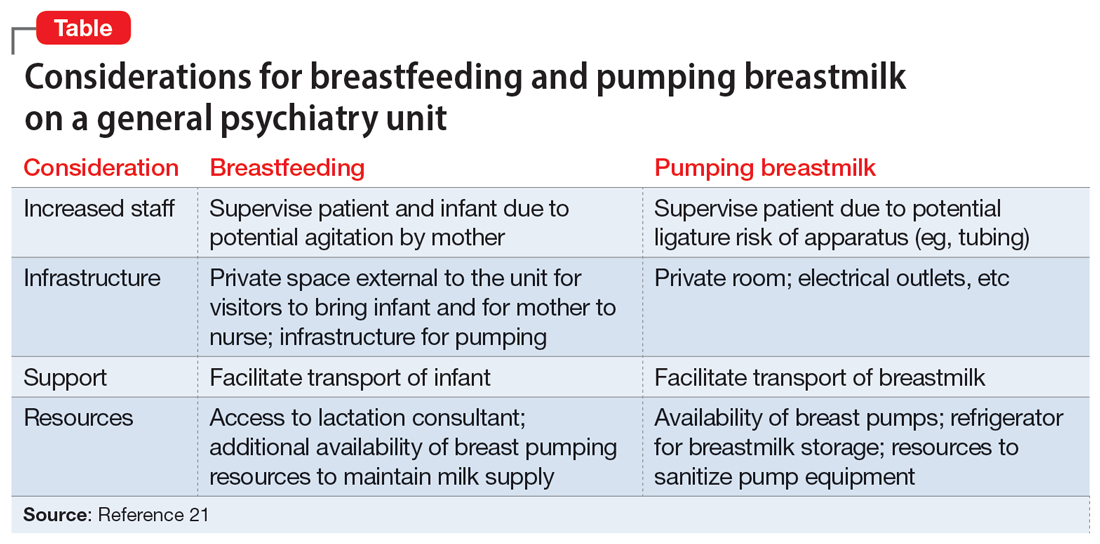
Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.
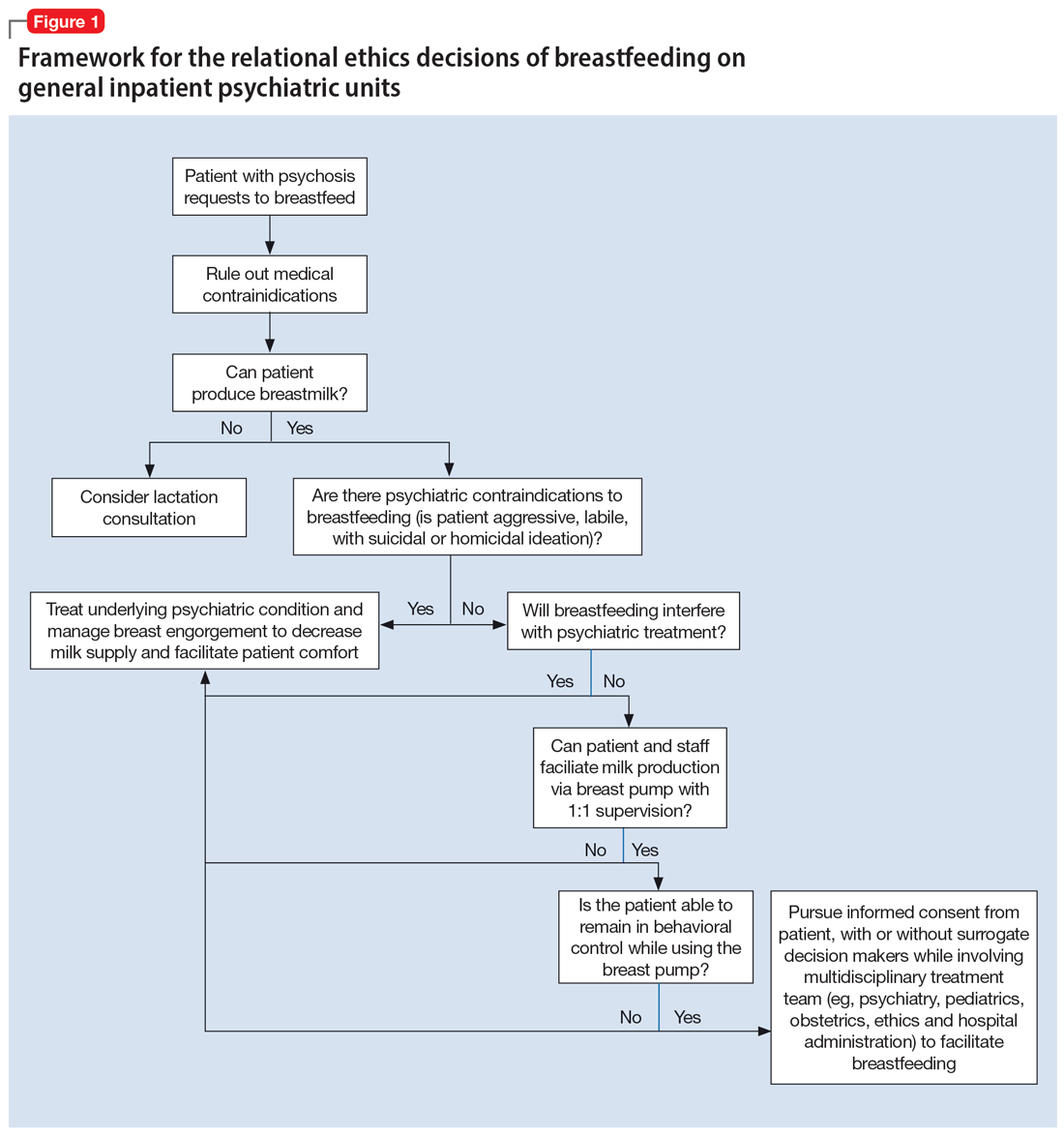
In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
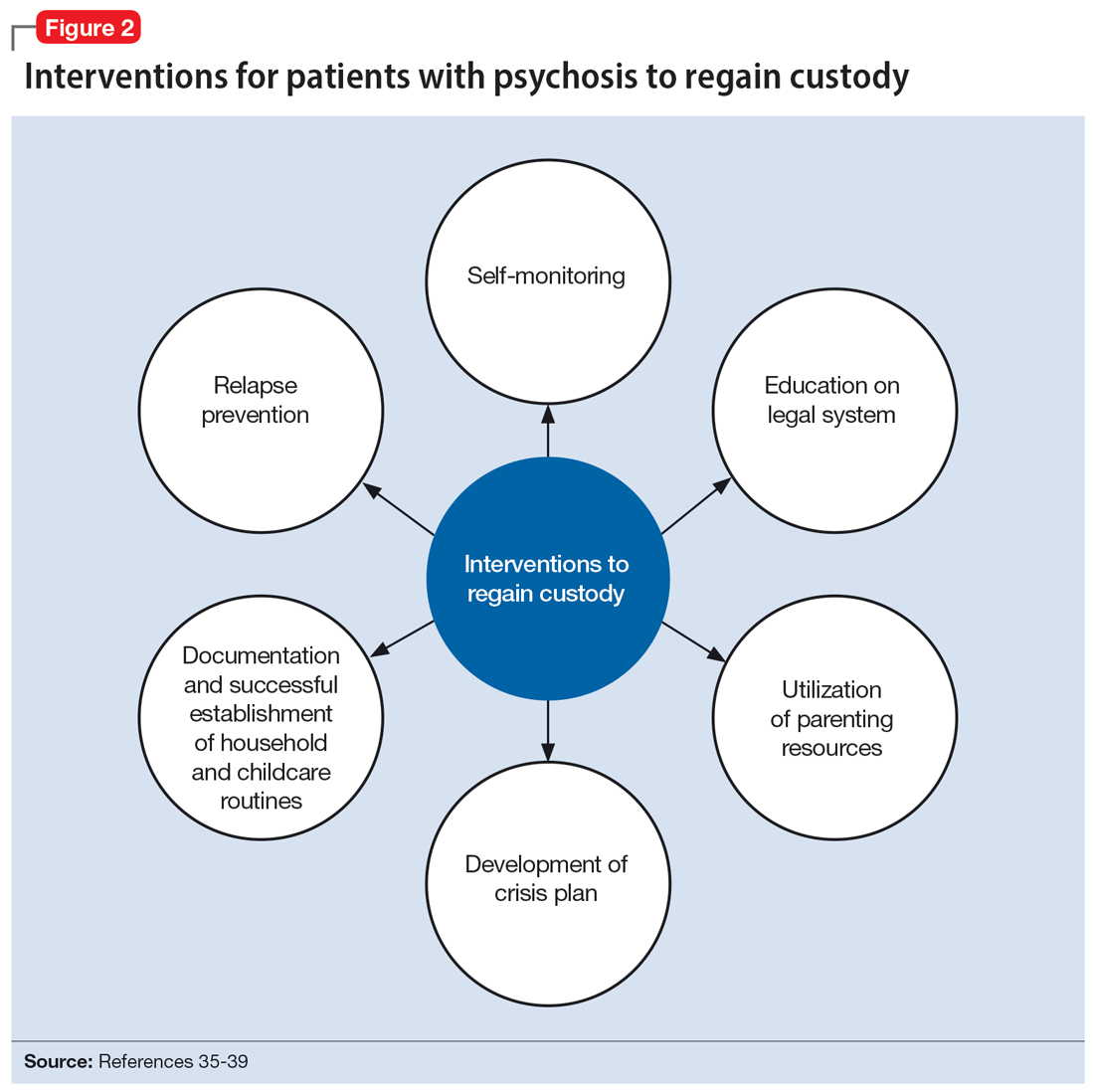
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.
Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Opioid use disorder in pregnancy: A strategy for using methadone
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.
Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
In the United States, opioid use by patients who are pregnant more than quadrupled from 1999 to 2014.1 Opioid use disorder (OUD) in the perinatal period is associated with a higher risk for depression, suicide, malnutrition, domestic violence, and obstetric complications such as spontaneous abortion, preeclampsia, and premature delivery.2 Buprenorphine and methadone are the standard of care for treating OUD in pregnancy.3,4 While a literature review found that maternal treatment with buprenorphine has comparable efficacy to treatment with methadone,5 a small randomized, double-blind study found that compared to buprenorphine, methadone was associated with significantly lower use of additional opioids (P = .047).6 This suggests methadone has therapeutic value for patients who are pregnant.
Despite the benefits of methadone for treating perinatal OUD, the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Patients typically take methadone once a day, and the dose is titrated every 3 to 5 days to allow serum levels to reach steady state.7 During pregnancy, there are increases in both the volume of distribution and medication metabolism secondary to increased expression of the cytochrome P450 3A4 enzyme by the liver, intestine, and placenta.8 Additionally, as the pregnancy progresses, the rate of methadone metabolism increases.9 Methadone’s half-life (20 to 35 hours) leads to its accumulation in tissue and slow release into the blood.10 As a result, patients with OUD who are pregnant often require higher doses of methadone or divided dosing, particularly in the second and third trimesters.11
In this article, we provide a strategy for divided dosing of methadone for managing opioid withdrawal symptoms in the acute care setting. We present 2 cases of women with OUD who are pregnant and describe the collaboration of addiction medicine, consultation-liaison psychiatry, and obstetrics services.
CASE 1
Ms. H, age 29, is G3P2 and presents to the emergency department (ED) during her fourth pregnancy at 31 weeks, 1 day gestation. She has a history of opioid, cocaine, and benzodiazepine use disorders and chronic hepatitis C. Ms. H is enrolled in an opioid treatment program and takes methadone 190 mg/d in addition to nonprescribed opioids. In the ED, Ms. H requests medically supervised withdrawal management. Her urine toxicology is positive for cocaine, benzodiazepines, methadone, and opiates. Her laboratory results and electrocardiogram (ECG) are unremarkable. On admission, Ms. H’s Clinical Opiate Withdrawal Scale (COWS) score is 3, indicating minimal symptoms (5 to 12: mild; 13 to 24: moderate; 25 to 36: moderately severe; >36: severe). Fetal monitoring is reassuring.
Ms. H’s withdrawal is monitored with COWS every 4 hours. The treatment team initiates methadone 170 mg/d, with an additional 10 mg/d as needed to keep her COWS score <8, and daily QTc monitoring. Ms. H also receives lorazepam 2 to 4 mg/d as needed for benzodiazepine withdrawal. Despite the increase in her daily methadone dose, Ms. H continues to experience opioid withdrawal in the early evening and overnight. As a result, the treatment team increases Ms. H’s morning methadone dose to 190 mg and schedules an afternoon dose of 30 mg. Despite this adjustment, her COWS scores remain elevated in the afternoon and evening, and she requires additional as-needed doses of methadone. Methadone peak and trough levels are ordered to assess for rapid metabolism. The serum trough level is 190 ng/mL, which is low, and a serum peak level is not reported. Despite titration, Ms. H has a self-directed premature discharge.
Five days later at 32 weeks, 2 days gestation, Ms. H is readmitted after she had resumed use of opioids, benzodiazepines, and cocaine. Her vital signs are stable, and her laboratory results and ECG are unremarkable. Fetal monitoring is reassuring. Given Ms. H’s low methadone serum trough level and overall concern for rapid methadone metabolism, the treatment team decides to divide dosing of methadone. Over 9 days, the team titrates methadone to 170 mg twice daily on the day of discharge, which resolves Ms. H’s withdrawal symptoms.
At 38 weeks, 5 days gestation, Ms. H returns to the ED after experiencing labor contractions and opiate withdrawal symptoms after she resumed use of heroin, cocaine, and benzodiazepines. During this admission, Ms. H’s methadone is increased to 180 mg twice daily with additional as-needed doses for ongoing withdrawal symptoms. At 39 weeks, 2 days gestation, Ms. H has a scheduled cesarean delivery.
Her infant has a normal weight but is transferred to the neonatal intensive care unit (NICU) for management of neonatal opioid withdrawal syndrome (NOWS) and receives morphine. The baby remains in the NICU for 35 days and is discharged home without further treatment. When Ms. H is discharged, her methadone dose is 170 mg twice daily, which resolves her opioid withdrawal symptoms. The treatment team directs her to continue care in her methadone outpatient program and receive treatment for her cocaine and benzodiazepine use disorders. She declines residential or inpatient substance use treatment.
Continue to: CASE 2
CASE 2
Ms. M, age 39, is G4P2 and presents to the hospital during her fifth pregnancy at 27 weeks gestation. She has not received prenatal care for this pregnancy. She has a history of OUD and major depressive disorder (MDD). Ms. M’s urine toxicology is positive for opiates, fentanyl, and oxycodone. Her laboratory results are notable for mildly elevated alanine aminotransferase, positive hepatitis C antibody, and a hepatitis C viral load of 91,000, consistent with chronic hepatitis C infection. On admission, her COWS score is 14, indicating moderate withdrawal symptoms. Her ECG is unremarkable, and fetal monitoring is reassuring.
Ms. M had received methadone during a prior pregnancy and opts to reinitiate treatment with methadone during her current admission. The team initiates methadone 20 mg/d with additional as-needed doses for ongoing withdrawal symptoms. Due to a persistently elevated COWS score, Ms. M’s methadone is increased to 90 mg/d, which resolves her withdrawal symptoms. However, on Day 4, Ms. M reports having anxiety, refuses bloodwork to obtain methadone peak and trough levels, and prematurely discharges from the hospital.
One day later at 27 weeks, 5 days gestation, Ms. M is readmitted for continued management of opioid withdrawal. She presents with stable vital signs, an unremarkable ECG, and reassuring fetal monitoring. Her COWS score is 5. The treatment team reinitiates methadone at 80 mg/d and titrates it to 100 mg/d on Day 7. Given Ms. M’s ongoing evening cravings and concern for rapid methadone metabolism, on Day 10 the team switches the methadone dosing to 50 mg twice daily to maintain steady-state levels and promote patient comfort. Fluoxetine 20 mg/d is started for comorbid MDD and eventually increased to 80 mg/d. Ms. M is discharged on Day 15 with a regimen of methadone 60 mg/d in the morning and 70 mg/d at night. She plans to resume care in an opioid treatment program and follow up with psychiatry and hepatology for her anxiety and hepatitis C.
A need for aggressive treatment
Given the rising rates of opioid use by patients who are pregnant, harmful behavior related to opioid use, and a wealth of evidence supporting opioid agonist treatment for OUD in pregnancy, there is a growing need for guidance in managing perinatal OUD. A systematic approach to using methadone to treat OUD in patients who are pregnant is essential; the lack of data surrounding use of this medication in such patients may cause overall harm.12 Limited guidelines and a lack of familiarity with prescribing methadone to patients who are pregnant may lead clinicians to underdose patients, which can result in ongoing withdrawal, premature patient-directed discharges, and poor engagement in care.13 Both patients in the 2 cases described in this article experienced ongoing withdrawal symptoms despite daily titration of methadone. This suggests rapid metabolism, which was successfully managed by dividing the dosing of methadone, particularly in the latter trimesters.
These cases illustrate the need for aggressive perinatal opioid withdrawal management through rapid escalation of divided doses of methadone in a monitored acute care setting. Because methadone elimination is more rapid and clearance rates increase during the perinatal period, divided methadone dosing allows for sustained plasma methadone concentrations and improved outpatient treatment adherence.9,14,15
Continue to: Decreasing the rate of premature discharges
Decreasing the rate of premature discharges
In both cases, the patients discharged from the hospital prematurely, likely related to incomplete management of their opioid withdrawal or other withdrawal syndromes (both patients had multiple substance use disorders [SUDs]). Compared to patients without an SUD, patients with SUDs are 3 times more likely to have a self-directed discharge.16 Patients report leaving the hospital prematurely due to undertreated withdrawal, uncontrolled pain, discrimination by staff, and hospital restrictions.16 Recommendations to decrease the rates of premature patient-directed discharges in this population include providing patient-centered and harm reduction–oriented care in addition to adequate management of pain and withdrawal.17
Impact of methadone on fetal outcomes
Approximately 55% to 94% of infants born to patients who are opioid-dependent will develop NOWS. However, there is no relationship between this syndrome and therapeutic doses of methadone.18 Moreover, long-term research has found that after adjusting for socioeconomic factors, methadone treatment during pregnancy does not have an adverse effect on postnatal development. Divided dosing in maternal methadone administration is also shown to have less of an impact on fetal neurobehavior and NOWS.19
Our recommendations for methadone treatment for perinatal patients are outlined in the Table. Aggressive treatment of opioid withdrawal in the hospital can promote treatment engagement and prevent premature discharges. Clinicians should assess for other withdrawal syndromes when a patient has multiple SUDs and collaborate with an interdisciplinary team to improve patient outcomes.

Bottom Line
The prevalence of opioid use disorder (OUD) in patients who are pregnant is increasing. Methadone is an option for treating perinatal OUD, but the physiological changes that occur in patients who are pregnant—coupled with methadone’s unique pharmacologic properties—may complicate its use. Using divided doses of methadone can ensure the comfort and safety of the patient and their baby and improve adherence and outcomes.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/cp.0305
- Townsel C, Irani S, Buis C, et al. Partnering for the future clinic: a multidisciplinary perinatal substance use program. Gen Hosp Psychiatry. 2023;85:220-228. doi:10.1016/j. genhosppsych.2023.10.009
Drug Brand Names
Buprenorphine • Buprenex, Suboxone, Zubsolv, Sublocade
Fentanyl • Abstral, Actiq
Fluoxetine • Prozac
Lorazepam • Ativan
Methadone • Methadose, Dolophine
Oxycodone • Oxycontin
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.
1. Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization – United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845-849.
2. Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139-151. doi:10.1016/S0889-8545(05)70362-4
3. Baumgaertner E. Biden administration offers plan to get addiction-fighting medicine to pregnant women. The New York Times. October 21, 2022. Accessed February 23, 2023. https://www.nytimes.com/2022/10/21/health/addiction-treatment-pregnancy.html
4. Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107 Suppl 1(0 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
5. Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5-27.
6. Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275-281. doi:10.1111/j.1360-0443.2006.01321.x
7. Substance Abuse and Mental Health Services Administration. Chapter 3B: Methadone. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families: Updated 2021. Substance Abuse and Mental Health Services Administration; August 2021. https://www.ncbi.nlm.nih.gov/books/NBK574918/
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519. doi:10.1053/j.semperi.2015.08.003
9. McCarthy JJ, Vasti EJ, Leamon MH, et al. The use of serum methadone/metabolite ratios to monitor changing perinatal pharmacokinetics. J Addict Med. 2018;12(3): 241-246.
10. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol Series No. 43. Substance Abuse and Mental Health Service Administration; 2005.
11. Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Createspace Independent Publishing Platform; 2018.
12. Balch B. Prescribing without data: doctors advocate for the inclusion of pregnant people in clinical research. Association of American Medical Colleges. March 22, 2022. Accessed September 30, 2022. https://www.aamc.org/news-insights/prescribing-without-data-doctors-advocate-inclusion-pregnant-people-clinical-research
13. Leavitt SB. Methadone Dosing & Safety in the Treatment of Opioid Addiction. 2003. Addiction Treatment Forum. Accessed November 28, 2023. https://atforum.com/documents/DosingandSafetyWP.pdf
14. McCarthy JJ, Leamon MH, Willitts NH, et al. The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med. 2015; 9(2):105-110.
15. DePetrillo PB, Rice JM. Methadone dosing and pregnancy: impact on program compliance. Int J Addict. 1995;30(2):207-217.
16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525. doi:10.1080/08897077.2019.1671942
17. McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66.
18. Jones HE, Jansson LM, O’Grady KE, et al. The relationship between maternal methadone dose at delivery and neonatal outcome: methodological and design considerations. Neurotoxicol Teratol. 2013;39:110-115.
19. McCarthy JJ, Leamon MH, Parr MS, et al. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606-610.

