User login
When is her pelvic pressure and bulge due to Pouch of Douglas hernia?
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).
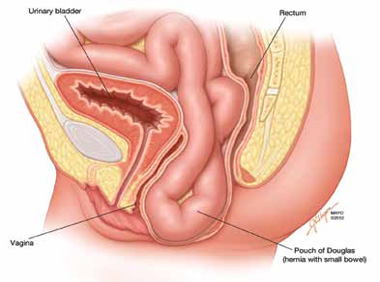
FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7
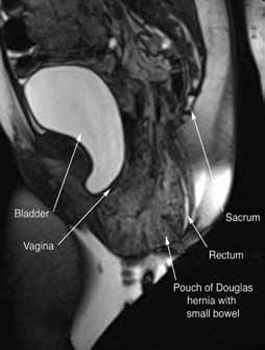
FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).
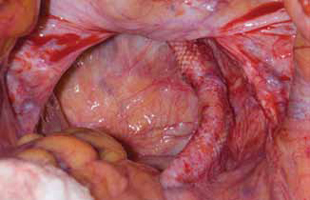
FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.
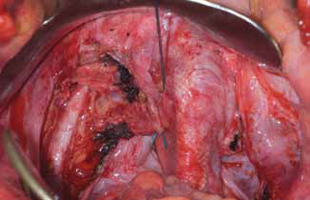
FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.
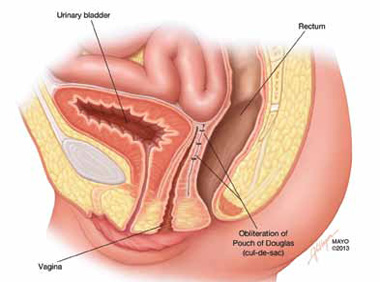
FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).

FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7

FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).

FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.

FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.

FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).

FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7

FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).

FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.

FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.

FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
IN THIS ARTICLE
Clinical pearls at physical exam
Treatment options

