User login
Anemia of chronic kidney disease: When normalcy becomes undesirable
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
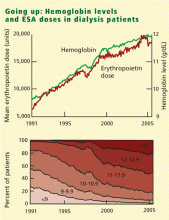
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
KEY POINTS
- ESAs reduce the need for blood transfusions and possibly improve quality of life.
- It is unclear if higher hemoglobin levels per se actually caused the adverse events in these trials. Event rates were highest in patients who responded poorly to ESAs.
- We concur with the FDA’s recommendation that the hemoglobin level be raised to no higher than 12 g/dL with ESAs in patients with chronic kidney disease or renal failure.
- Transient excursions of the hemoglobin level above 12 g/dL should not be a cause for panic. Rather, the next ESA dose should be reduced.